Applications of Coumarins as Cardiovascular and Anti-Cancer Agents: A Short Review-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Coumarins (2H-1-benzopyran-2-one), an important class of heterocyclic compounds, and its derivatives can be found in many natural or synthetic drug molecules and possess versatile bioactivities making them important molecules for medical practitioners and medicinal chemists. Coumarins have found applications in drug molecules especially related to oncogenic and cardiovascular dysfunctions. Cardiovascular diseases and cancer are lifestyle diseases that have become plague to the society. This review highlights the bioactivities of Coumarins towards these life threatening diseases.
Keywords: Coumarins; Anti-cancer; Cardio-vascular; Anti-coagulant; Anti-oxidant
Introduction
Coumarins (1), are naturally occurring fragrant compounds belonging to the benzopyrone family and have been used in perfumes since 1882 [1]. Coumarins have also attracted the interest of biochemists and medicinal chemists for a long time due to their diverse biological activities [2]. Over and above the discoveries made by isolation of Coumarins from the hundreds of species of plants and other organisms, synthetic analogues have availed numerous and varied Coumarins. These natural as well as synthetic derivatives of Coumarins have drawn much attention due to their diverse pharmacological activities [3]. Various moieties when combined with Coumarins can produce same or different effects with different potencies.
Many Coumarins and their synthetic derivatives thus exist and exhibit anti-coagulant, anti-tumor, anti-viral, anti-inflammatory, anti-oxidant, anti-microbial and enzyme inhibitory properties [2,4-7] (Figure 1).
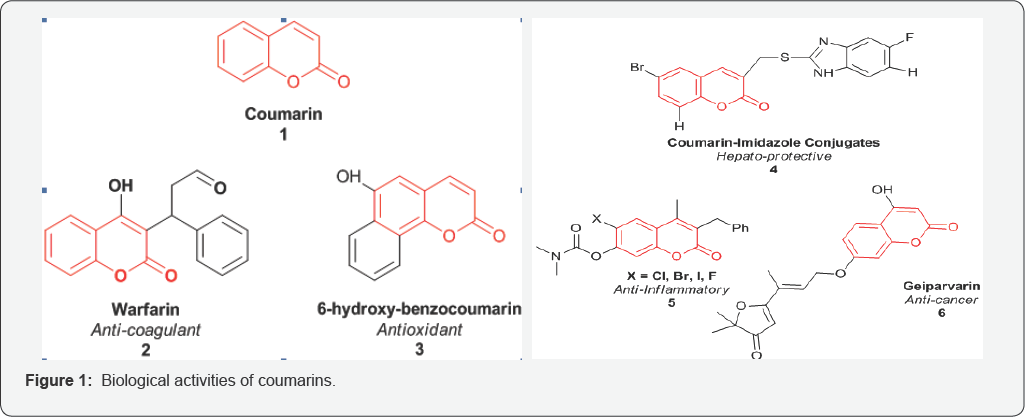
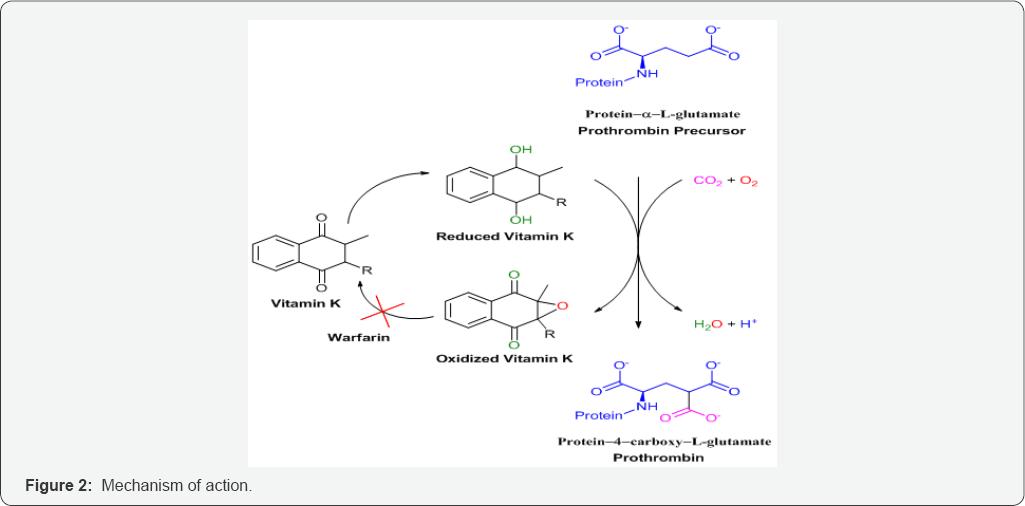
Coumarins are naturally occurring anticoagulants. They act as a competitive inhibitor of vitamin K epoxide reductase, an enzyme that recycles vitamin K. Vitamin K epoxide reductase (VKOR) is an integral membrane protein that catalyzes the reduction of vitamin K 2,3-epoxide and vitamin K to vitamin K hydroquinone, a cofactor required for the gamma-glutamyl carboxylation reaction (Figure 2). The VKOR decreases the concentration of reduced vitaminK, which reduces the rate of vitamin K-dependent carboxylation and leads to under-carboxylated, inactive vitamin K-dependent proteins. It is proposed that an active site disulfide needs to be reduced for the enzyme to be active. VKOR uses two sulfhydryl groups for the catalytic reaction and these two sulfhydryl groups are oxidized back to a disulfide bond during each catalytic cycle [8-10].
Cardiovascular Agents
Coumarin based anticoagulants are effective in the treatment and prevention of arterial and venous thrombosis. Nowadays, the main clinical use of coumarins based anticoagulants is the prevention of stroke or systemic embolism in patients with arterial fibrillation, which is one of the most common cardiac disorders [8,11].
Coumarins are also known to possess anti-oxidant property, which attributes to direct scavenging of reactive oxygen and nitrogen species (ROS and RONs). One important mechanism through which this is achieved is by donating hydrogen to free radicals in its reduction to nonreactive species. Addition of hydrogen would remove the odd electron feature that is responsible for radical reactivity [12]. It is also observed due to inhibition of enzymes like xanthene oxidase, which are responsible for formation of these free radicals [13,14]. Coumarins thus also exhibit positive cardiovascular effects partially due to their antioxidant activity. Many coumarins have been shown to relax the contractions of vascular smooth muscle induced by mediators like sympathomimetics nor epinephrine and phenylephrine or high doses of potassium chloride (KCl) [11]. Methoxy derivative of Osthole extracted from Cnidium monnieri (C. monnieri) exhibited vasodilatory effect on rabbit corpus cavernosum. Similar activity was observed for some butyrolactone derived Coumarins [15].
The basic mechanism for vascular relaxations is by controlling the intracellular concentration of calcium ions. Coumarins are proposed to block the entry of calcium ion by inhibiting its release from the sarcoplasmic reticulum or stop the in-flow of calcium through voltage dependent calcium channels and receptor- operated calcium channels located in the membrane of smooth muscle cells [11].
Anti-Cancer Agents
Coumarin and its derivatives have also been used for treatment of variety of cancer. Coumarin derivatives when used in conjunction with other modes of treatment may provide better results [16]. The current work also attempts to highlight the cytotoxic activities of coumarins on different types of cancers (Figure 3).
Breast Cancer
Coumarin derivatives, 7-hydroxycoumarin 7, and its substituted analogues have shown to decrease cell proliferation in case of breast cancer [17]. 5,6-benzo-[a]-pyrone, or 1,2-benzopyrone substituted derivatives have shown to decrease the lymphedema through stimulation of proteolysis by tissue macrophages [18]. It has been observed that substitution at C-3 position via an amide linkage has shown promising activity against melanoma and breast cancer cell lines [19]. A family of coumarin-3-(N-aryl) carboxamides (8) (Figure 3) were prepared and evaluated as cytotoxic agents against SKBr3 and BT474 breast cancer cell lines as well as normal HFLs (human lung fibroblasts that do not over express the ErbB-2 and EGFR). The SAR (structure activity relationship) studies showed that the substitutions on the Coumarin ring and also on the N-phenyl moiety can strongly affect the cytotoxic profile of the compounds [20].
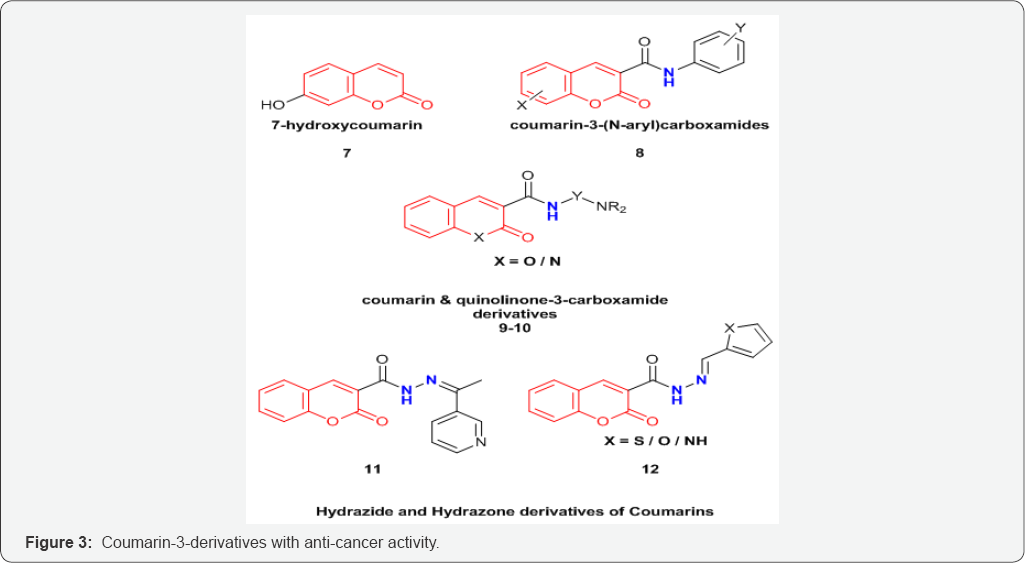
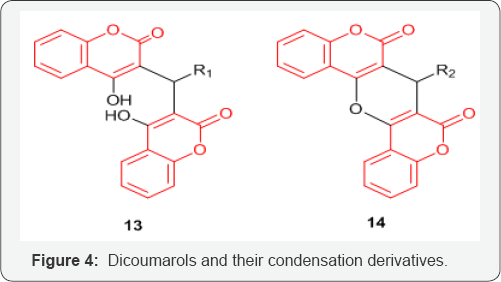
Series of Coumarin and quinolinone-3-carboxamide derivatives 9 and 10 (Figure 3) were synthesized and examined for their anticancer activities against several cancer cell lines including prostate cancer cells DU145 and PC3, colon cancer HCT15, breast cancer MCF7, ovarian cancer IGROV1, liver cancer SKHep1, and leukemia cancer HL60(TB). The above study by Matiadis et al. [21] demonstrated that Coumarin derivative containing N-(8-aminooctyl) side chain [X=O, Y=(CH2)8, and R=H] had the highest inhibitory effects against DU145 prostate cancer cells (GI50<10mM). Mohareb et al. [22] synthesized several hydrazide hydrazone derivatives including coumarin 11,12 (Figure 4). The study conducted by Mohareb et al. [22] showed that effect of 11, on the in vitro growth of three human tumor cell lines namely breast adenocarcinoma (MCF7), non-small cell lung cancer (NCIH460) and CNS cancer (SF268) indicated that the best inhibitory activity was against NCIH460 with IC50 value of 10mM [22].
Lung Cancer
Coumarin and 7-hydroxycoumarin inhibited cell growth by inducing cell cycle arrest in the G1 phase in all the lung carcinoma cell lines [23]. Series of dicoumarol derivatives 13 and their condensation products with acetic anhydride 14 (Figure 4) have shown promising activity against SK-LU-1, SPC-A-1 and 95D human lung cancer cell lines [24]. Amino side chains or alkoxy derivatives of coumarins have shown to induce apoptosis in the human lung (A549) cancer cell line [25].
Anti-Leukemic Activity
5-methoxy-6,7-methylenedioxy coumarin and 5-(3-methyl- 2-butenyloxy)- or 6,7-methylenedioxy coumarin inhibited proliferation and induced differentiation in human leukemia U-937 cells [26]. The Complexes of cerium(III), lanthanum(III) and neodymium(III) with 4-methyl-7-hydroxy coumarin showed marginal cytotoxic activity against transformed leukemic cell lines (P3HR1 and THP-1) as compared to the inorganic salts [27].
Pancreatic Cancer
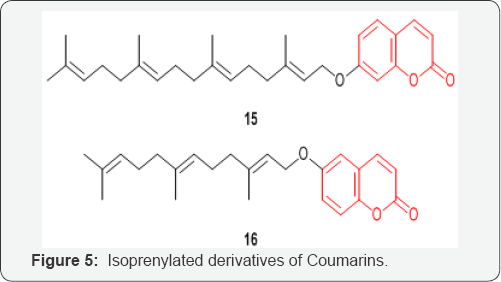
A series of isoprenylated Coumarins 15 and 16 (Figure 5) were designed, synthesized, and evaluated against human pancreatic adenocarcinoma cell line PANC-1 yielding a series of potent and selective cytotoxic agents under nutrient deprived conditions [28].
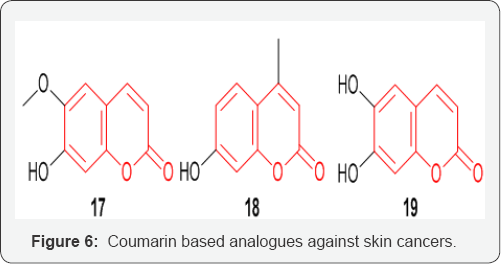
Coumarins and their substituted analogues have also proven themselves as potential candidates to treat skin cancer. Scopoletin 17 an alkaloid separated from ethanolic extract of the medicinal plant, Gelsemium sempervirens (Fam: Loganiaceae) has been reported to have anti-cancer potential [29] (Figure 6) Bhattacharya et al. [30] reported that 4-methyl-7-hydroxycoumarin 18 compound has a great potential as a possible anti-cancer drug, particularly in polycyclic aromatic hydrocarbon induced skin cancer [30]. Jeon & Young-Joo et al. [31] demonstrated that esculetin 19 induced apoptosis in the HMM cells by down regulating Sp1 protein levels. Their results suggest that esculetin may be a potential anti-proliferative agent that induces apoptotic cell death in G361 HMM cells [31].
Cervical Cancer
Cervical cancers are one of the most common gender-specific cancers affecting women worldwide. The effects of coumarin on cell viability, cell cycle arrest and induction of apoptosis were investigated in human cervical cancer HeLa cells by Chuang & Jing-Yuan et al. [32]. It was observed that coumarin was cytotoxic with an IC50 of 54.2microM, induced morphological changes, and caused G0/G1 arrest and apoptosis.
Miscellaneous
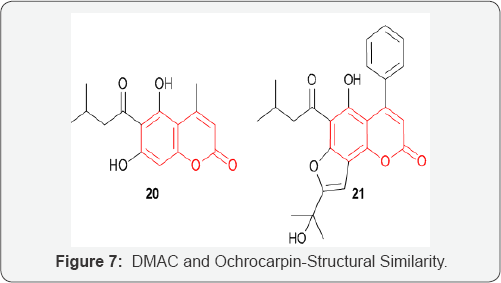
The antitumor activity of a novel coumarin derivatives 5,7-dihydroxy-4-methyl-6-(3-methylbutanoyl)-Coumarin (DMAC) 20, on colorectal carcinoma was investigated. DMAC was initially investigated due to its structural similarity to the cytostatic agent ochrocarpin B 21 (Figure 7). Ochrocarpin B was extracted from the bark of Ochrocarpos punctatus, along with several other coumarins and benzophenone derivatives, all exhibiting cytotoxicity against an ovarian cancer cell line [33]. Indeed, a large number of synthetic and natural Coumarins act as cytotoxic agents [34]. Treatment of colon cancer HCT116 and LoVo cells with DMAC resulted in substantial proapoptotic activity. The combination of DMAC 38 treatment with the established anticancer drugs 5-FU and CPT-11 enhanced their therapeutic efficacies. Structure activity studies on DMAC related compounds led to the conclusion that substitution at 6-position is crucial for inducing cell apoptosis and a phenyl group at 4-position presumably enhances the bioactivity [35]. New cytostatic agents based on the DMAC structure could therefore lead to interesting new therapeutic valuable compounds.
Conclusion
The life altering events like cardiovascular diseases or cancer are a plague to society and affect many families across the globe. This review highlights the versatility of coumarins molecules and their multiple biological activities, thus highlighting their importance and tremendous scope for future medical practitioners and medicinal chemists.
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/open-access.php
For more articles in Open Access Journal of Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment