Pediatric Clinical CT Cases-Juniper Publishers
Abstract
Five clinical pediatric CT cases; the first case has dysnea, respiratory distress, cardiomegaly and gallop. Echocardiography showed dilated left ventricle with impaired contractility EF 20%, FS 10%. CT angiography showed absent left main coronary artery, anomalous origin of the LCX and LAD by separate ostia from the left side of the main pulmonary artery, diffuse dilatation of the RCA, Many dilated inter-coronary collaterals (adult type of ALCAPA). The second case has dysnea, recurrent pneumonia, respiratory distress, cardiomegaly and gallop. Echocardiography demonstrated dilated left ventricle with impaired contractility EF 22%, FS 10%, suspected ALCAPA. CT revealed anomalous origin of the LAD from the left side of the main pulmonary artery, left circumflex arises from the left coronary sinus of Valsalva and runs its normal course, mild diffuse dilatation of the RCA (ALCAPA). The third case has 9 months old female, recurrent pneumonia, feeding difficulties, poor weight gain, cardiomegaly and accentuated S2. Echocardiography revealed dilated RV, severe TR, moderate sized secundum ASD and pulmonary hypertension. CT showed average sized main pulmonary artery continuous with its left branch, absent right pulmonary artery, indirect MAPCA supplying the right lung, diminished right lung volume. The forth case 20 months old female, dysnea, cyanosis, left upper limb weakness, and systolic murmur. Echocardiography showed TOF. CT showed average sized confluent main pulmonary artery and it's both branches, abnormal origin of the left subclavian artery from the left pulmonary artery, right sided aortic arch. The fifth case has 18 months old female, 6 months following surgical closure of VSD and epicardial pacemaker insertion, dysnea, respiratory distress, pansystolic murmur. Echocardiography revealed abnormal flow directed from the aorta to the RV, suspected ruptured sinus of Valsalva. CT showed residual VSD between the LVOT and RV cavity just below the Tricuspid valve.
Introduction
SComputerized Tomography CT Angiography of the heart and blood vessels could be considered now is an important tool for the diagnosis and management of congenital heart diseases whether before or after surgical or interventional management as well as follow up and detection of post-management complications. In the last two decades, computed tomography (CT) scan has emerged as valuable non-invasive cardiovascular diagnostic tool capable of producing informative pictures, providing unique anatomic and functional information not available by any other diagnostic modality currently available; as well as for the assessment of the anatomy of the extra-cardiac structures.
Case Report 1 (Dilated Cardiomyopathy in a Four Years Old Girl)
Introduction
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) syndrome, also known as Bland- White-Garland syndrome, is a rare congenital abnormality that affects 1 of every 300,000 live births [1] and accounts for 0.25%- 0.5% of all congenital heart defects [2]. It usually manifests as an isolated defect, but in 5% of cases it may be associated with other cardiac anomalies such as atrial septal defect, ventricular septal defect, and aortic coarctation [3].
ALCAPA syndrome results in the “coronary steal” phenomenon, in which a left-to-right shunt leads to abnormal left ventricular perfusion. ALCAPA syndrome is one of the most common causes of myocardial ischemia and infarction in children. If left untreated, up to 90% of patients with ALCAPA syndrome die within the 1st year of life [4]. In patients who live to adulthood, ALCAPA syndrome may cause myocardial infarction, left ventricular dysfunction and mitral regurgitation, or silent myocardial ischaemia, which can lead to sudden cardiac death. Early diagnosis and prompt surgical intervention with the aim of restoring a two-coronary-artery circulatory system have excellent results and lead to gradual myocardial recovery.
Clinical picture
By history; four years old female, presented by dysnea, growth delay and respiratory distress grade II to III, by examination; cardiomegaly and gallop was noted.
Echocardiography
It revealed dilated cardiomyopathy, markedly dilated left ventricle with markedly impaired contractility Ejection Fraction 20%, Fractional Shortening 10%.
CT angiography on the heart and blood vessels
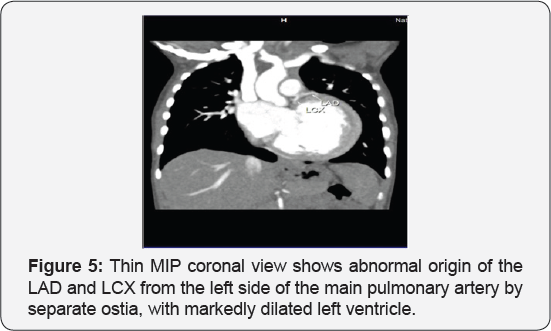
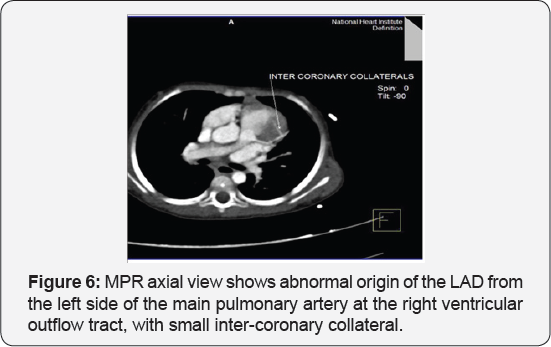
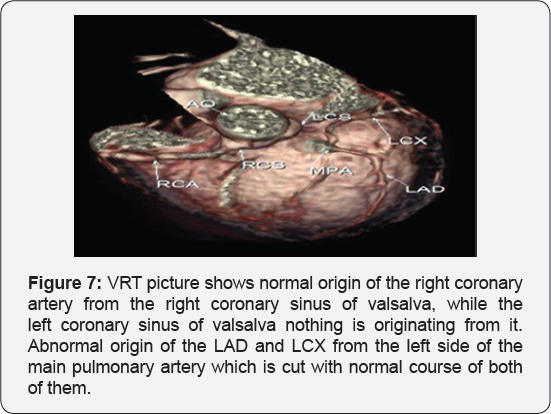
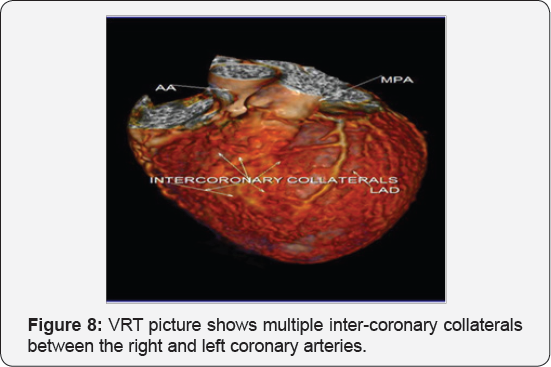
It revealed a case of dilated cardiomyopathy showing: Absent left main coronary artery, Anomalous origin of the Left Circumflex and Left Anterior Descending arteries by separate ostia from the left side of the main pulmonary artery, Diffuse dilatation of the Right Coronary Arteries, Many dilated inter-coronary collaterals. A picture of adult type of ALCAPA (Figure 1).
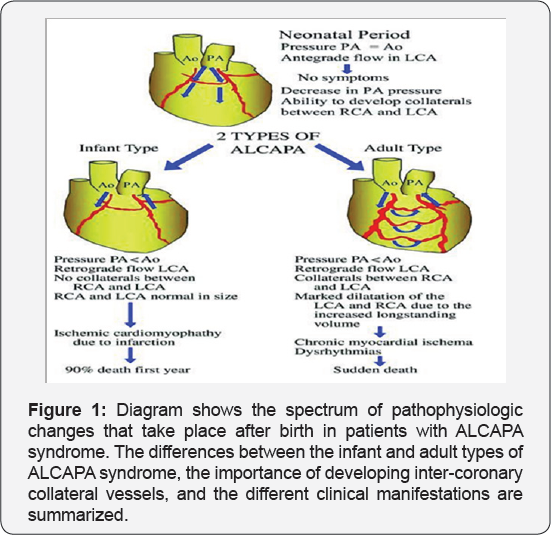
Pathophysiology
In fetal and early neonatal life, the origin of the left coronary artery (LCA) from the pulmonary artery is well tolerated because pulmonary arterial pressure equals systemic pressure, which leads to antegrade flow in both the anomalous LCA and the normal right coronary artery (RCA) [5].
Soon after birth, when pulmonary arterial pressure decreases, flow in the LCA decreases and then reverses, which leads to myocardial ischaemia and infarction. The extent of acquired collateral circulation between the RCA and LCA during the critical period, when pulmonary arterial pressure gradually decreases, determines the extent of myocardial ischaemia (Figure 1). Patients with well-established collateral vessels have the adult type of the disease, and those without collateral vessels have the infant type. Both types of the disease have different manifestations and outcomes.
Infant type: The onset of symptoms usually occurs about 8 weeks after birth. There is little or no coronary collateral development. When the reversal of flow in the LCA is established, a limited blood supply to the left ventricular myocardium leads to congestive heart failure and mitral insufficiency secondary to myocardial infarction [4,6]. The most important differential diagnosis in this age group is dilated cardiomyopathy [1]. Infants typically present with a failure to thrive, profuse sweating, dysnea, pallor, and atypical chest pain while eating or crying. Without surgical repair, rapid death ensues in up to 90% of patients within weeks or months of birth [4].
Adult type: As pressure decreases in the pulmonary circulation and as flow reverses in the LCA, the LCA fails to supply the myocardium and "drains" fully oxygenated blood into the main pulmonary artery. Thus, there is preferential blood flow into the low-pressure pulmonary circulation rather than into the highresistance myocardial circulation. This left to-right shunt is known as the steal phenomenon. To survive beyond infancy, patients with ALCAPA syndrome develop significant collateral circulation from the RCA to the LCA. However, often it is not sufficient to supply the left ventricle, especially in the sub-endocardial region; chronic left ventricular sub-endocardial ischaemia ensues. As a result, patients may develop malignant ventricular dysrhythmias. These patients are at risk for sudden cardiac death, which occurs in 80%-90% of cases [7-11]. Patients may be asymptomatic, or they may present with mitral insufficiency, ischemic cardiomyopathy, or malignant dysrhythmias, which lead to sudden death [4] (Figure 2-8).
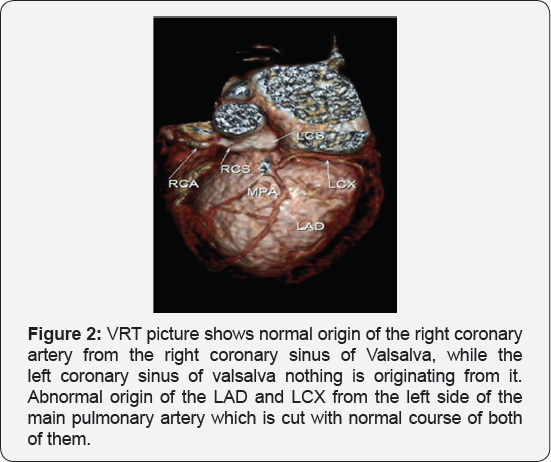
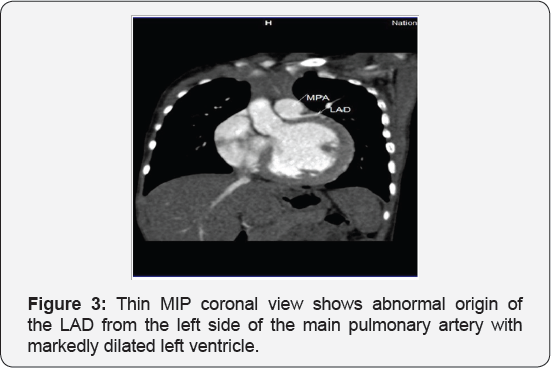
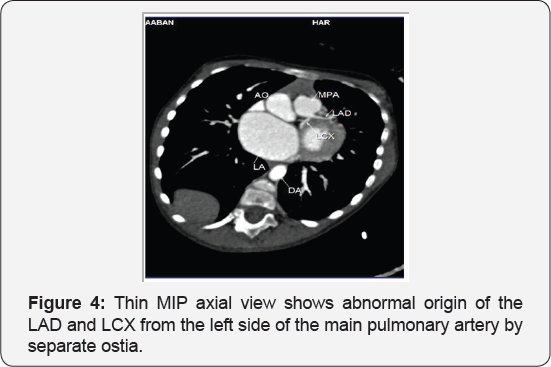
Case Report 2 (Dilated Cardiomyopathy in Ten Months Old Girl)
Clinical picture
By history; ten months old female, presented by dysnea, recurrent chest infections, growth delay and respiratory distress grade II to III, cardiomegaly and gallop by examination. It revealed dilated cardiomyopathy, markedly dilated left ventricle with markedly impaired contractility EF 22%, FS 10%, suspected ALCAPA.
CT angiography on the heart and blood vessels
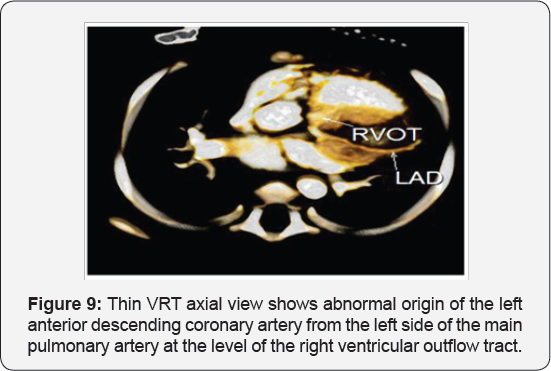
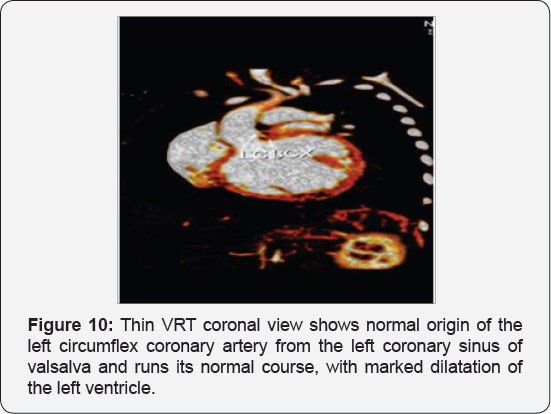
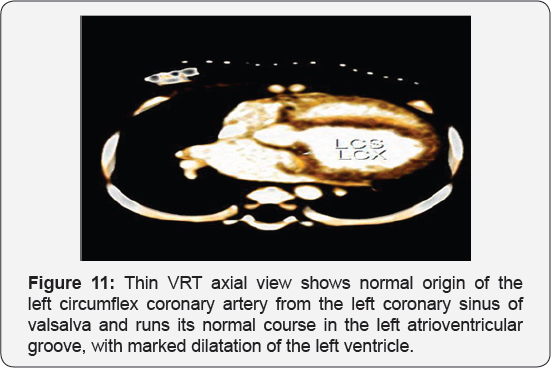
It revealed a case of dilated cardiomyopathy showing; Anomalous origin of the LAD by a separate ostium from the left side of the main pulmonary artery, while the left circumflex arises from the left coronary sinus of valsalva and runs its normal course, with Mild diffuse dilatation of the RCA (A picture of ALCAPA) (Figure 9-12).

Case Report 3 (ASD with Severe Pulmonary Hypertension)
Introduction
Isolated absence of right pulmonary artery is a rare congenital lesion with a diverse clinical presentation. This defect was first reported by Fraentzel in 1868. Pool et al. [12] studied 78 cases with absence of either of the branch pulmonary arteries and found 32 cases (~40%) without any additional cardiac lesion. Fifty-six patients in their series had additional structural cardiac problems such as Tetralogy of Fallout (21%), patent ductus arteriosus (PDA; 14%) and septal defects (9%). Isolated absence of RPA was associated with pulmonary hypertension in 19-44% of the patients in different case series [13]. Isolated absence of RPA frequently presents in infancy with PHT and congestive cardiac failure [14]. A high index of suspicion is required to diagnose this condition in infancy [15].
Clinical picture
Nine months old female presenting by recurrent chest infections, respiratory distress, feeding difficulties and poor weight gain, cardiomegaly and accentuated second heart sound by examination.
Echocardiography
Dilated right ventricle, severe Tricuspid regurgitation, moderate sized secundum atrial septal defect and severe pulmonary hypertension.
CT angiography on the heart and blood vessels
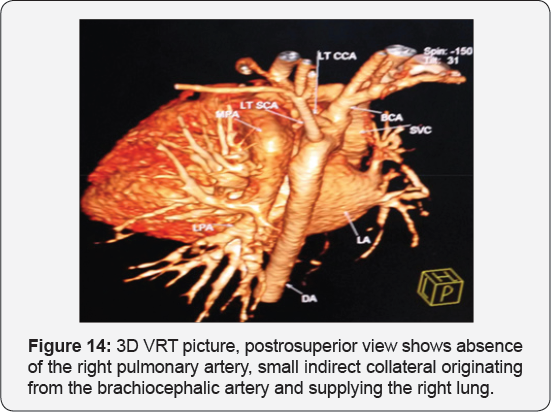
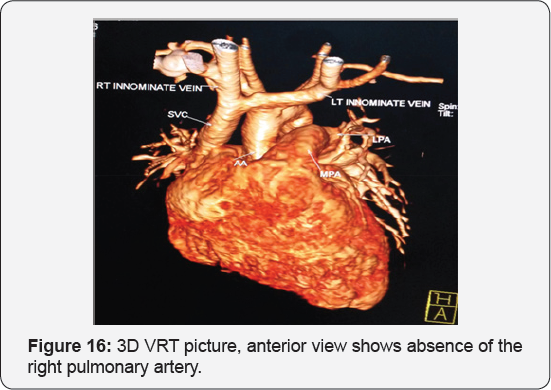
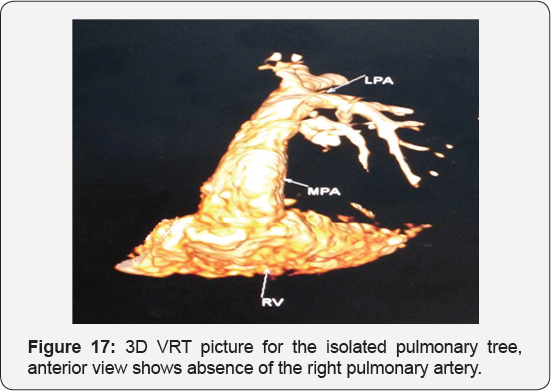
It revealed a case of moderate sized secundum atrial septal defect showing: Average sized main pulmonary artery continuous with its left branch, absent right pulmonary artery, indirect major aorto-pulmonary collateral originating from the brachiocephalic artery and supplying the right lung, diminished right lung volume with minimal pleural effusion.
Discussion
Two types of presentations are described. The first presentation is the one seen in infants, where they usually present with congestive cardiac failure and PHT [16,17]. The other presentation is in older patients, who usually do not develop pulmonary hypertension and do not have manifest heart failure. They present with exercise intolerance (18-40%), hemoptysis (20%) or are incidentally detected during chest radiography [18]. The diagnosis of isolated absence of RPA is based on history, clinical evaluation and imaging. A high index of suspicion is needed to make the diagnosis. In infancy, the signs can be subtle and can be easily missed [14,15]. The electrocardiogram is usually normal in patients with uncomplicated isolated absence of pulmonary artery (without PHT), whereas it shows right ventricular dominance in cases associated with PHT [16]. Chest X-ray may show an absent hilar shadow, absence of the left or RPA, reduction of pulmonary vascular markings on the right side (~60% cases), a small hemithorax and intercostal bone space, shrunken affected lung and a shift of the mediastinal structures to the affected side with contralateral lung hyperinflation [14,19]. A cardiac MDCT scan and magnetic resonance imaging (MRI) can confirm the diagnosis and delineate the pulmonary artery anatomy along with delineating MAPCA if present.
Conclusion
Any infant with unexplained PHT should be thoroughly evaluated for the possibility of isolated unilateral absence of pulmonary artery (UAPA). Differential vascularity on the chest X-ray may often be a clue for the diagnosis. Isolated absence of RPA is a rare entity. In infancy, it presents with respiratory distress and severe PHT. A high index of suspicion is needed to diagnose the entity. Differential vascularity in the chest X-ray can give a clue. MDCT scan and MRI can confirm the echocardiographic diagnosis and delineate the pulmonary artery anatomy. The surgical treatment plan depends on the presence of a good-sized hilar pulmonary artery and the presence of MAPCA. Early surgical or hybrid intervention may improve survival. Medical management includes symptomatic treatment for congestive cardiac failure and long-term pulmonary vasodilator for PHT. Infants with severe PHT are difficult to treat and have an unfavorable outcome (Figure 13-17).


Case Report 4 (Tetralogy of Fallot with Upper Limb Weakness)
Clinical picture
Twenty months old female, presented by dysnea, cyanosis and growth delay, weakness of the left upper limb (reported by the parents; for which receiving physiotherapy), right ventricular enlargement, systolic murmur, unequal radial pulsations, intact femoral pulses, blood pressure was 91/62 in right upper limb and 61/33 in the left upper limb with left upper limb weakness, hypotonia and hyporeflexia by examination.
Echocardiography
It revealed tetralogy of fallout with good size of pulmonary arteries.
CT angiography
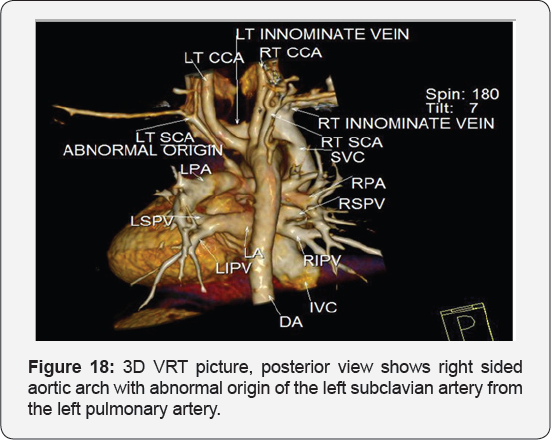
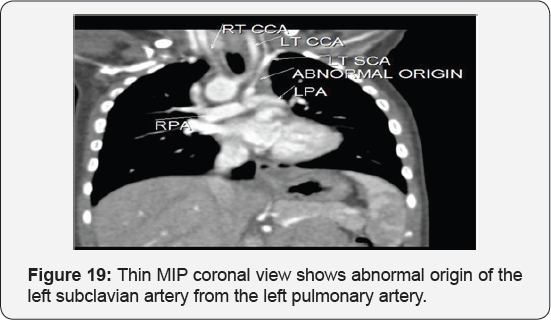
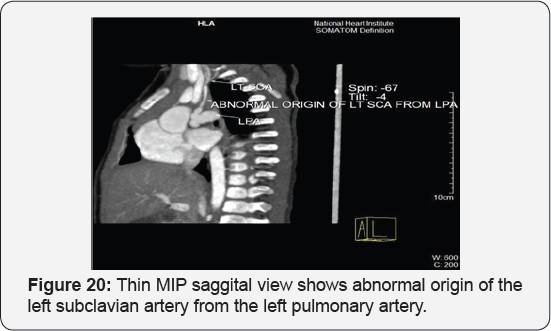
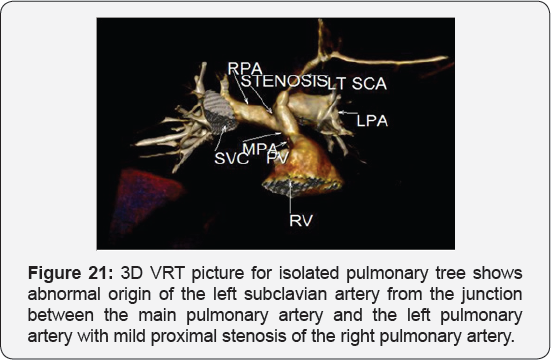
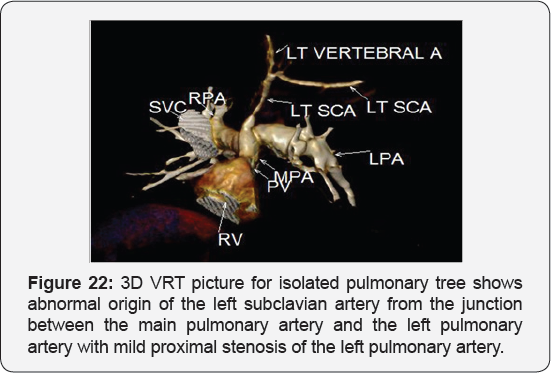
It revealed: A case of tetralogy of Fallout showing: average sized confluent main pulmonary artery and its branches, McGoon's ratio 2.2/1, bifurcational stenosis of the left and right pulmonary arteries, right sided aortic arch with mirror image branches, abnormal origin of the left subclavian artery from the left pulmonary artery at its junction with the main pulmonary artery.
Theoretical background
Definition: Left subclavian artery isolation is an uncommon anomaly in which the right sided aortic arch is associated with anomalous origin ofthe LSCA from the pulmonary artery via ductus arteriosus or ligamentum arteriosum without communication with the aorta Weinberg et al. [13].
Embryology: The development of the aortic arch and its branches occurs during the third week of gestation. A common arterial trunk arises from the primitive heart and divides into six paired aortic arches that fuse and form bilateral dorsal aortae which, in turn, fuse caudally into the descending aorta. The persistence or regression of these arches may lead to various aortic arch anomalies Edwards et al. [6]. Edwards' embryologic model of aortic arch malformation explains this LSCA isolation by the interruption of the left aortic arch at two locations: between the left carotid and subclavian arteries, or between the ductus arteriosus and the left dorsal aortic root Edwards et al. [6].
Associated anomalies: The isolation of the LSCA is commonly associated with 22q11 deletion and congenital heart diseases e.g. Tetralogy of Fallot, Double-outlet right ventricle and D-transposition of the great arteries [20].
Clinical presentations: The condition is usually asymptomatic and discovered during the evaluation of the associated cardiac anomalies or when reduced blood pressure is detected in the left arm. Left arm ischaemia, including pain, weakness, coldness, and reduced limb length, may be present in about 15% of cases (cases not associated with patent ductus arteriosus [12]).
Treatment: surgical reimplantation of the left subclavian artery and ligation or device closure of the PDA if present.
Conclusion: Anomalies of the aortic arch and its branches should be considered in cases of unequal radial pulse, unequal blood pressure in arms, upper limb weakness or ischaemia [12,16-23] (Figure 18-22).
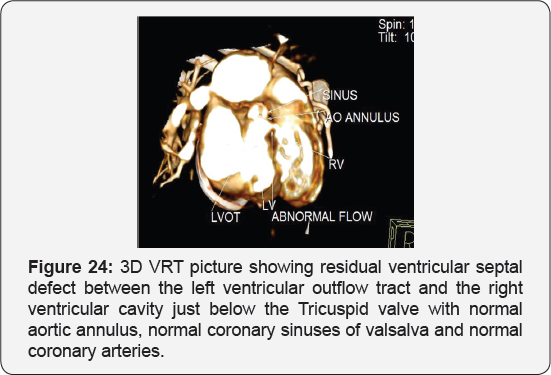
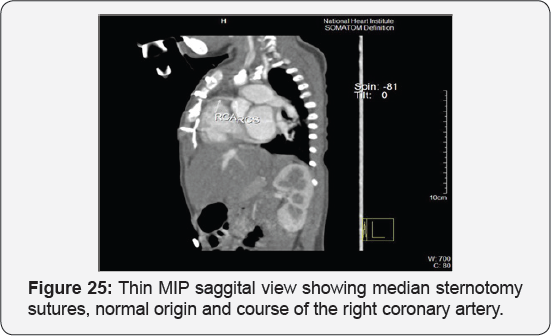

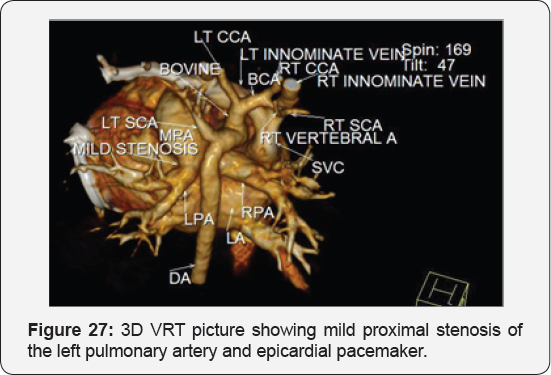
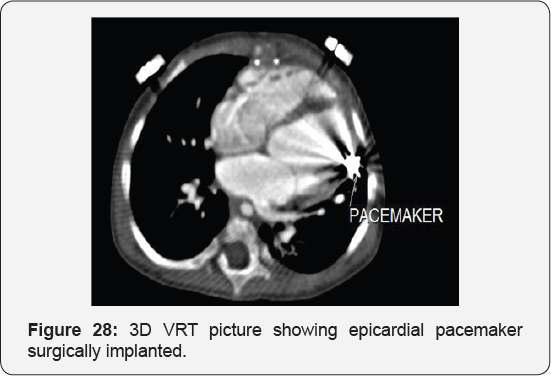
Case Report 5 (Postoperative Residual VSD)
Clinical picture
Eighteen months old female, presented 6 months following surgical closure of ventricular septal defect followed by surgical epicardial pacemaker implantation by dysnea and respiratory distress grade II to III, harsh pansysteolic murmur over the left parasternal area by examination,
Echocardiography
It revealed abnormal flow directed from the aorta to the right ventricle, suspected ruptured sinus of valsalva.
CT angiography on the heart and blood vessels
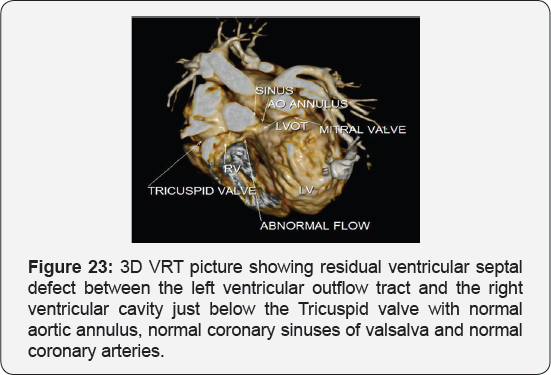
It revealed status post surgical VSD closure and epicardial pacemaker insertion, showing: Residual ventricular septal defect between the left vtricular outflow tract and the right ventricular cavity just below the Tricuspid valve, with normal aortic annulus, normal coronary sinuses of valsalva and normal coronary arteries with Mild proximal stenosis of the left pulmonary artery (Figure 23-28) [24-30].
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/open-access.php
For more articles in Open Access Journal of Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment