Proton Pump Inhibitors and Clopidogrel: What arethe Clinically Important Interactions between Them?-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Background: Antiplatelet therapy is the hallmark therapy for patients with coronary artery disease status post percutaneous coronary intervention (PCI) with stenting. Millions of patients are subjected to such intervention and therapy annually. However recently, attention has been raised against the use of PPIs regarding its potential to decrease the efficacy of clopidogrel. Even the U.S. Food and Drug Administration (FDA) has issued warnings regarding the related use of these medications.
Objective: We have evaluated multiple studies in an effort to shed light into the ambiguity of concomitant use of PPIs and clopidogrel in relation to increase risk for myocardial infarction (MI).
Method: A web-based literature search was conducted through PubMed and Scholar using the keywords "proton pump inhibitors,” "Myocardial infarction,” and "clopidogrel.” Of the available results, 78 relevant studies were reviewed and summarized.
Conclusion: After evaluating the 78 relevant studies, we conclude that there is significant lack of evidence regarding drug-drug interaction between PPIs and clopidogrel. Several studies have shown that PPIs alter the inhibitory effect of clopidogrel, but are deemed to be of low quality and increased bias. For this reason, a randomized clinical trial is pivotal to help resolve the controversy of concomitant use of PPIs and clopidogrel in relation to increase risk for Myocardial infarction. For clinicians with concerns of such interaction we recommend an alternative option like H2-receptor antagonists or maintaining a time gap between the administration of PPI and clopidogrel.
Keywords: Proton pump inhibitors; Myocardial infarction; Clopidogrel
Introduction
The H+/K+ ATPase physiology of the gastric tissue was known in 1970s, since then a counter regimen was under preparation against acidic environment of stomach [1]. Initially, timiprazole was considered due to its anti-secretory activity but later, by optimization, omeprazole was accepted as the final product for medical use [2]. The groups of these anti-secretory compounds are popularly known as Proton Pump Inhibitors (PPIs). They soon became one of the top prescribed over-the-counter drugs worldwide. Most of the PPIs are the derivatives of benzimidazole although the newer principal compound, imidazopyridine is more promising and may replace the current widely prescribed PPIs [3].
Proton pump inhibitors are commonly prescribed for gastro esophageal reflux disease (GERD), a disease that is rapidly increasing worldwide. Although the prevalence on geographic distribution is quite variable, North America stands uppermost on the utilization list with 18.1%-27.8% and East Asia lowest with less than 10% [4]. A probable explanation is the 'diet shift' amongst the Americans that contributes to this figure. It is expected that an increase in disease burden will be observed in coming years, especially in North America which has contributed a raise of 5% prevalence, annually [5].
The demand of PPIs is directly proportional to the increasing prevalence, making it third most sold medication in US, annually making a $13 billion market [6]. Another independent report from IMS Health stated a $6 billion market in 2012 from a single PPI brand, Nexium [7]. The market dynamics are quite favorable for the pharmaceutical companies to invest in this pool for a lucrative future. It is possible that more licenses for production of PPIs will be issued internationally to counter the increasing prevalence of GERD. But are we sure that this huge market share is not blinding the adverse effects or that the companies are reluctant to share the post-market surveillance reports? The fact is that evidence based side effects of PPIs have yet been documented.
Despite its good safety profile, a recent statistical study published from Stanford University involving the data-mining of the previous consumers of PPIs have established an alarming situation for the patients. The study has stated PPIs causing myocardial infarction (MI) independently, a shocking statement for a drug which is used in high numbers and for nearly half a century [8]. How this study will be addressed is a big question for the boards of the healthcare professionals as well as by the regulatory authorities.
Physiology, Drug Interactions and Side Effects
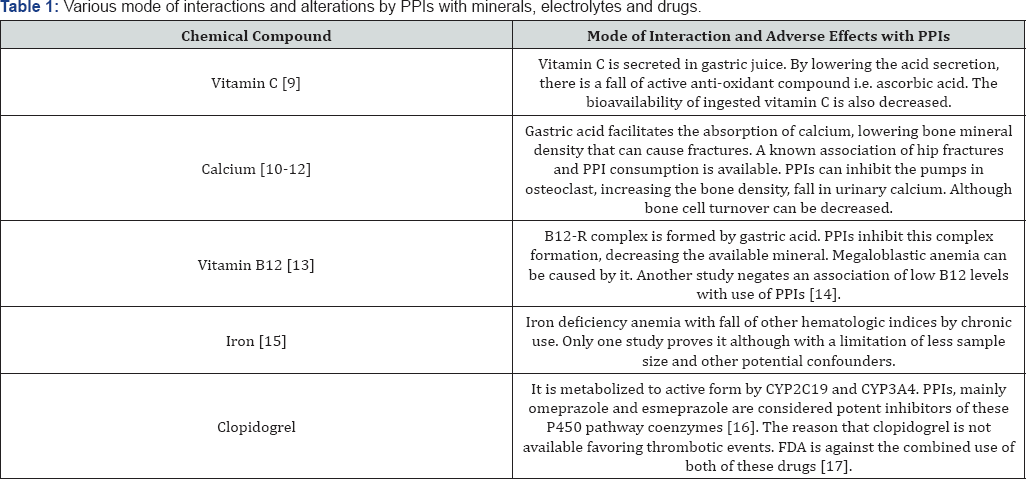
PPIs inhibit H+/K+ ATPase, which prevents the secretion of H+, responsible for HCl in stomach. By inhibiting this channel, there is decreased acid formation, eventually the reflux will be devoid of acid. This will prevent from heartburning and adversity of the exaggerated physiological process [3]. So far, it is considered as one of the safest drug groups because the documented studies presenting adverse effects are not very representative for the populations. Although altering the pharmacodynamics of clopidogrel and hampering in some electrolyte physiology are a few commonly known effects. One important side effect is its inhibiting capacity of cytochrome P450 enzymes. Any drug involving this pathway must be used with extra caution. The interactions of PPIs with other chemical compounds are collected in Table 1 [9-17].
Clopidogrel, MI and PPIs
Coronary disease or MI constitutes the leading cause of death in western world and will keep on increasing in coming years [18]. Previously, the condition used to be diagnosed by electrocardiograms, which have almost now been replaced by cardiac biomarkers with Troponin I and T, the most specific markers to identify the disease. Troponin does not provide an accurate diagnosis but enables a comprehensive look into prognosis. With these advancements, better treatment algorithms have evolved in past years to prevent subsequent episodes of MI [19-21]. The American Heart Association devised an algorithm to treat this disease and its variants along with cerebrovascular accidents with clopidogrel, a pivotal medicine preventing thrombotic events. It is indicated in ST-elevated ischemia, stable or unstable angina and percutaneous coronary interventions [22,23].
It has been proven that PPIs hamper the clopidogrel metabolism which reduces the bioavailability of active ingredient that provides anti-platelet function, promoting thrombi, eventually MI. Clopidogrel is an oral medication with main indication in central or peripheral vascular events. It works by inhibiting ADP receptors that are responsible for aggregation of the platelet clumping [24]. PPIs are inhibitors of hepatic cytochromes hindering in the normal anti-platelet physiology of clopidogrel. In 2009, after a clinical cohort study was released, a clear warning was issued by the US FDA suggesting PPIs should not be used with clopidogrel.
Fontes-Carvalho R [25] carried a cross over trial of clopidogrel users, first with omeprazole and after a washout period, pantoprazole. The efficacy of clopidogrel was maintained in the second trial, the drawn conclusions favored pantoprazole over omeprazole. A single dose of pantoprazole showed significant lowering of acid amongst the fast metabolizing genotype CYP2C19 [26]. A study carried by Siller-Matula [27] from Vienna on 300 patients, including coronary artery disease (CAD) and percutaneous coronary intervention (PCI) concluded that pantoprazole and esomeprazole are not associated with impaired response of clopidogrel based upon the platelet reactivity index. Some safer evidence about lansoprazole is available as well from Small DS, suggesting its non-interactive behavior with clopidogrel or prasugrel [28]. If PPIs are an absolute need, pantoprazole may be considered for use because of its minimal effects on CYP enzymes, unlike others in this group [29].
Amid safe and unsafe PPIs and mixed conclusions, a study comprising of an extensive cohort of PCI and ACS patient from 3 states in the US, with an extended timeline of 5 years, concluded that there is no evidence of PPI-clopidogrel interaction, or if evidence exists it is less than 20%. The study can be objectionable but it has to be considered that the sample size of clopidogrel users is huge (n= 18,565) to establish this new side of debate [30]. Despite such favorable points for PPIs, an objection was raised by Potter [31] demanding the clarification of the developed score adjustment, cohort development and some non-compliance issues of the included sample units.
These studies have provided evidence that the whole group of PPIs cannot be discarded overnight for causing direct or indirect interaction with clopidogrel and the FDA needs to once again probe into the matter as revised version of the warning may be needed.
MI Independent of Clopidogrel use amongst the PPI Users
The association of PPI with myocardial infarction is not only related to interaction with Clopidogrel. Charlot et al. [32] conducted a large retrospective included aspirin treated patients, excluding Clopidogrel treated patients, and showed an increase of cardiovascular effects in patients treated with PPI when compared with patients treated with H2 receptor blocker. Charlot et al. [33] in a cohort study which included nationwide unselected population showed that PPI alone was associated with increase of myocardial infarctions; the authors concluded that the believed explanation for the correlation of PPI and Clopidogrel use with myocardial infarction is explained by the presence of confounders [33]. CREDO (Clopidogrel for the Reduction of Events during Observation) trial showed that patients treated with PPI, regardless if they received Clopidogrel or placebo, had an increase in cardiovascular events when compared to patients without PPI use [34]. Clopidogrel and the Optimization of Gastrointestinal Events Trial (COGNET) did not show any difference in rate of cardiovascular events in patients treated with or without PPI. Despite that the trial conflicted with results of previous studies, this trial was underpowered and resulted in a wide hazard ratio for cardiovascular events [35].
Discussion
Though we quoted a few studies which somehow correlate that MI is associated with PPI users whether they were clopidogrel users or not, we must consider that the facts established were either simple observations or drawn from patients already with multiple diseases. There are limitations to the documented knowledge and it must be remembered that we cannot neglect these observations. It is clear that safety of the drug requires long time post market surveillance. Nonetheless the chances of bias cannot be completely ignored. The observation of MI in those taking PPIs along with clopidogrel is understandable, but the studies suggesting an independent association is a query still to be answered. Either clinical trials or extensive cohorts can only be clinically and statistically relevant.
For more Open Access Journals in Juniper Publishers please
click on: https://juniperpublishers.com
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php
For more Open
Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment