The Novel Anti-Thrombotic Drug with No-Bleeding Excess-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Background and purpose: All anti-thrombotic drugs presently available cause increased bleeding, a most undesirable effect. Our aim was to test a novel anti-thrombotic drug [Th001] which, theoretically should not suffer from this disadvantage.
Experimental approach: The trial design was a double-blind, placebo controlled, paired crossover, with 99% power for showing a difference in skin bleeding time. n=48 Caucasian patients, age 69.0±6.6 years, 38 male, 10 female, with stable atherothrombotic disease and other stable chronic diseases, treated with aspirin and statin. There was no effect on bleeding time (p =0.9729) nor on tests of haemostasis, (Ultegra point of care aggregation test and flow cytometry). The mean platelet count rose from 234.43±67.264 to 246.43±84.14x10-9 per litre, p=0.024.
Conclusion: Th001 therapy appears to be a safe method for potential treatment and prevention of occlusive arterial thrombosis with no risk of increased bleeding.
Keywords: Arterial thrombus growth inhibition; Coronary thrombosis; Thrombotic stroke; Peripheral arterial disease; Normal haemostasis
Introduction
This clinical study was performed in 2006-2007, on a drug for which the preclinical results were published, when it was entitled ICI 170809 [1]. Publication submission for this clinical study has been delayed for a variety of reasons, viz:
- Failure to attract sufficient academic grants to develop the drug for clinical use.
- Death of Professor Gabrielle Hawksworth, who was the senior pharmacologist on the study. This publication is a memorial to her.
- Lack of effective intellectual property for an old drug that lost its molecular patent in 2003.
- Delay in forming a company that sought such intellectual property, which was a long process ending in the grant of a US patent of use in December 2015.
- In spite of favourable reception from several referees, journals have declined publication in view of the age of the data. Contrary to that view is the opinion of [2], in the book 'Bad Pharma', namely the insistence that all results of drug trials should be published, regardless of age.
Serotonin is involved in platelet-rich thrombus growth in diseased, stenosed arteries (with increased shear rates and endothelial damage). Serotonin is not involved in the initial platelet activation, aggregation and fibrinogen binding to form a haemostatic layer when normal tissue is damaged; such tissue contains no serotonin. Conventional drug discovery for anti platelet drugs requires that there should be a measurable inhibitory effect upon conventional platelet function tests which all measure haemostatic platelet function; such drugs will inevitably cause bleeding. Drug discovery for an anti-thrombotic agent that does not cause bleeding should not have these effects. Arterial thrombosis tends to occur at arterial narrowings, pointing to the probability that the convective acceleration, generating increased shear stress, is the predominant stimulus to platelet-rich thrombus growth at these sites. When platelet deposition occurs in an arterial stenosis, serotonin is released from the dense granules of the platelets and activates more platelets through the 5HT2A receptors, leading to a platelet-to- platelet positive feedback reaction (snowball)-the platelet-rich thrombus growth. Therefore, platelet-rich thrombus growth can be differentially blocked by ant agonising the platelet serotonin receptor, of the 5HT2A subtype, leaving haemostatic function intact. That this occurs with the 5HT2A antagonist Th001 (formerly ICI 170809) is known from the work of McAuliffe et al. [1]. This was shown in the "gold standard" method for this purpose -the Folts model of the anaesthetised dog with damaged and stenosed coronary artery [3]. These animals, which are studied during a thoracotomy, show no increased surgical bleeding upon administration of the ICI 170809, now known as Th001 (ArteclereTM). There is thrombus dispersal that is not due to a direct action of Th001, but to the effect of natural thrombolysis when thrombus growth is abolished. Although this measurement cannot be performed in man, there is no reason to think that there is a great difference between man and dog. The Folts model has always reliably predicted the efficacy of antiplatelet drugs in man [3].
We report the results of an academic study funded by grant to the University of Aberdeen, carried out on consenting patients at Aberdeen Royal Infirmary, monitored by the Research and Development department, and approved by MHRA. The patients were stable with controlled atherothrombotic arterial disease, either coronary or peripheral, attending as out-patients. The results failed to demonstrate any significant effects of Th001 upon a number of indices of haemostasis.
Methods
Patients
The study, including patient information sheet, invitation letter to patients, informed consent form, and GP information letter were approved by Grampian Region Ethics Committee, conformed to BJP guidelines. Patients with inclusion criteria were invited to participate by letter. This was a real patient situation because of minimal exclusion criteria. Inclusion- patients previously diagnosed with either coronary arterial disease, defined as ≥70% stenosis on angiography or peripheral arterial disease, with ankle/brachial pressure ratio <0.9 these patients were treated with aspirin and a statin. Exclusions-taking warfarin or clopidogrel, leg ulceration and rest pain, females of child bearing age, and patients receiving serotonin re-uptake inhibitors. 46 patients were recruited with ischaemic heart disease plus 10 patients with peripheral vascular disease. A total of 48 patients, after exclusions completed the study. We report the completed studies on 48 Caucasian patients, age 69.0±6.6 years, 38 male, 10 female, with stable athero-thrombotic disease treated with aspirin and statin. The entire study was carried out from 2006 to 2007.
The co-morbidities included hyperlipidaemia (treated) 23, hypertension(treated) 20, diabetes mellitus 11, renal disease 9, hepatic insufficiency 8, left ventricular dysfunction 7, hypothyroidism 6, chronic obstructive pulmonary disease 5, iron deficiency anaemia 5, diverticular disease; duodenal disease 4, hearing loss 4. Other diseases in fewer numbers of patients each were: haematuria, prostatic disease, urinary incontinence, back pain, macrocytic anaemia, asthma, glaucoma, haemorrhoids, hiatus hernia, impotence, Meniere's disease, osteoporosis, retinopathy, sciatica, ankylosing spondylitis, anxiety and depression, aortic aneurysm, aortic stenosis, cervical spondylosis, Charcot foot, Crohn's disease and macular degeneration. These diseases were considered to be stable by the patients’ physicians. The past histories of these patients included bacterial meningitis, basal cell carcinoma, cervical polyp, colonic polyp, CVA, pneumonia, pulmonary tuberculosis, numerous and various operations.
The study design was double-blind, placebo controlled cross over. Subjects attended the Cardiology Research suite for five visits: control pre-treatment baseline, on course 1 for 2 weeks, washout for 2 weeks, on course 2 for 2 weeks, washout for 2 weeks. Course 1 was randomized to placebo or Th001 10mg bd and reversed for course 2. The randomization and preparation of courses was carried out by an independent agency at Ninewells Hospital, Dundee. All participants in the study -patients, staff and investigators were blinded to the randomization code. At each visit, physical measurements were: clinical examination plus electrocardiogram (ECG), including heart rate, QT interval and QT interval corrected for heart rate (QTc), followed by:
Venepuncture: Venous blood was collected into vacutainers by venepuncture with 19G butterfly and adaptor. The first 5ml of blood was used for full blood count, and subsequent samples collected in order for the platelet tests and others.
Platelet aggregation tests: Platelet aggregation was measured using the RPFA Ultegra [4], with Verify now cartridges for aspirin and GPIIB/IIIA (Accumetrics, San Diego, CA). This is a well-validated, point-of-care system which tests the ability of activated platelets in whole blood to bind fibrinogen-coated micro-beads contained within the cartridge test wells. Blood is stimulated with either thrombin-receptor activating peptide (TRAP) in the GPIIB/IIIA assay, or arachidonic acid (AA) in the aspirin assay. Results are reported as platelet aggregation units (PAU) for the GPIIB/IIIA assay and aspirin reaction units (ARU) for the aspirin assay (>550 ARU indicates a poor- or nonresponse to aspirin. Our inter-assay variation is 5.8% for RPFA- Aspirin and 5.0% for RPFA-GPIIB/IIIA.
Platelet activation: This was assessed as fibrinogen binding to resting and stimulated platelets by whole blood flow cytometry [5], as previously validated [6]. Briefly, citrate- anticoagulated blood was diluted ten-fold with HEPES-Mg buffer within 10 minutes of blood sampling. Diluted blood was incubated with FITC-conjugated rabbit anti-human fibrinogen antibody (Dako Cytomation, Denmark), for 20 minutes and the reaction was stopped by dilution with cold PBS. In order to assess the responsiveness of platelets to agonist stimulation, samples were incubated for 5min with 10-5M or 10-6M adenosine diphosphate (ADP) (Sigma Chemical Co. Ltd, UK), 105M serotonin (5-HT) or a combination of 10-6M ADP and 10-5M 5-HT, before addition of antibodies. Samples were analysed on the Coulter EPICS-XL flow cytometer (Beckman Coulter Inc, Ca, USA). Platelets were identified and gated in a separate sample by forward and side-scatter and by positive labelling with FITC- conjugated CD61 antibody, which binds specifically to platelet glycoprotein IIIa (Dako Cytomation, Denmark). Results are expressed as percentage of platelets positive for fluorescent antibody staining for fibrinogen.
Plasma Th001 assay: Samples were blinded and batch processed, after the end of the study, by a staff scientist, in order that the code could not be broken. Plasma was prepared by centrifugation of citrate-anticoagulated blood at 2500xG for 10 minutes, within 1 hour of venepuncture, frozen, stored at-80 °C until assay. Plasma Th001 concentrations were determined using a Thermo Surveyor-TSQ Quantum LC-MS/MS system (Hemel Hempstead, UK). Briefly, 50μl of plasma were precipitated by vortex mixing with 200μl of acetonitrile containing 100ng/ ml imipramine as the internal standard and centrifuged at 10,000rpm for five minutes. 5.0μl of the supernatant was injected onto the chromatograph. Imipramine and Th001 (formerly ICI 170809 and obtained from ICI Pharmaceuticals) were resolved on a Nucleosil 5μ ODS column (150 x4.6mm) with a mobile phase of 20% 0.1% triethylamine, pH4.0 and 80% acetonitrile at a flow rate of 1ml/min, which was split to allow 200μl/min through the mass spectrometer. The first four minutes after injection were diverted to waste. Both compounds were analysed using an electrospray source in positive ion mode and quantification performed on the SRM transition fragments m/z 337.1-m/z 292.1 for Th001 and m/z 281.1-m/z 86.1 for imipramine. Calibration standards and quality control samples were prepared by spiking blank plasma with known concentrations of Th001 and processing as described for the patient samples. Calibration curves were linear up to 1000ng/ ml with a lower limit of quantification of 1ng/ml.
Compliance: Compliance was determined after the end of the study by examination of the plasma Th001 concentrations. Plasma Th001 concentration assays showed 100% compliance, but the concentrations during Th001 administration were 89.1±59.0ng/ml. In 6 patients who received Th001 as course 1 and achieved the highest peak concentrations, a little Th001 was still detectable during course 2 (placebo) period.
Skin bleeding time: Skin bleeding time was performed, contra-laterally from venepuncture. A suitable area of forearm was shaved if necessary and cleaned with alcohol swab. A sphygmomanometer cuff was inflated on the upper arm to 40mmHg. One incision was made with Surgicutt bleeding device (Elitech, Hertfordfshire), and a stopwatch was started. Filter paper (Whatman No.1) was applied to the droplet at 30 second intervals, avoiding touching the skin, until bleeding stopped. Bleeding time was recorded to the nearest 30 seconds. These measurements were made by a trained nurse, blinded to the phase of the treatments.
Other observations: Measurements made by Aberdeen Royal Infirmary (ARI) routine full blood count, Clinical Haematology Laboratory; urea and electrolytes (U&Es), creatinine, Liver Function tests (LFTs) and blood glucose, Clinical Biochemistry Laboratory. Prothrombin fragments F1+2 by the University Platelet Laboratory.
Statistical analysis: We tested variables from placebo and Th001 patient data for Gaussian normality distribution by the Kolmogorov and Smirnov method. Normally distributed data sets were then analysed by paired t test, and non-normally distributed data were analysed by Wilcoxon paired signed rank test. Two weekly interval measurements of platelet count were analysed by repeated measures ANOVA. In Stat software (Graph Pad, San Diego) was used on a MacIntosh Apple computer (iMac).
Results
Th001 assays
We were aiming at 100μg/kg body wt. As there are 70ml blood per kg body wt, this is equivalent to 7μg/litre, i.e., 7ng/ ml. During Th001 administration, plasma Th001 concentration was 91.47±60.09ng/ml. The levels achieved are an order of magnitude higher than planned.
- In all patients during the Th001 period, Th001 was detected.
- In all patients taking placebo before Th001, no Th001 was detected.
- In 30 patients, Th001 was detected in the washout period following the Th001 period.
- In 6 of these patients who took Th001 before placebo, Th001 was detected during the placebo period, i.e., carried over from the Th001 period.
- In 4 of these patients, Th001 was detected in the washout period following the placebo period.
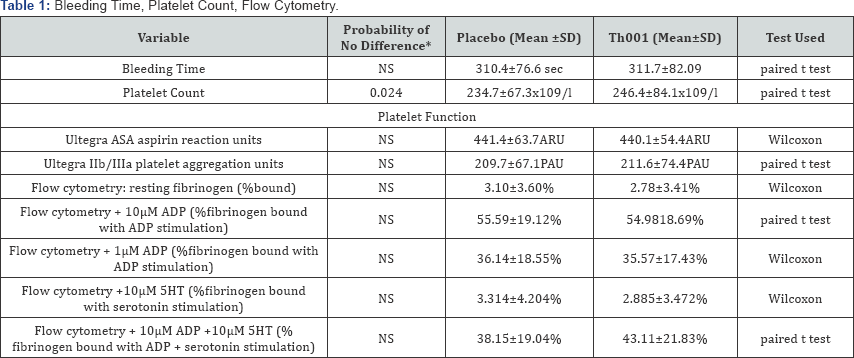
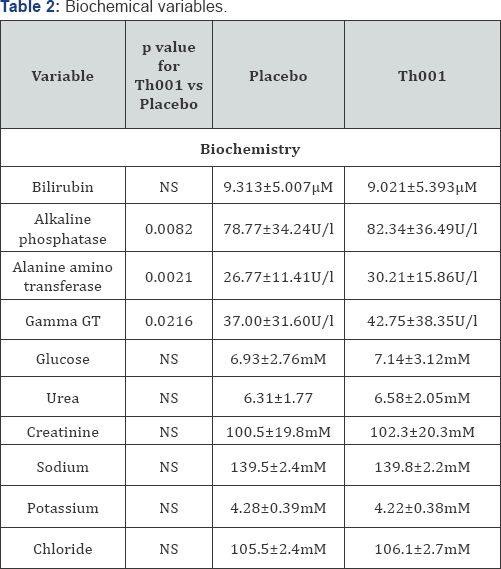
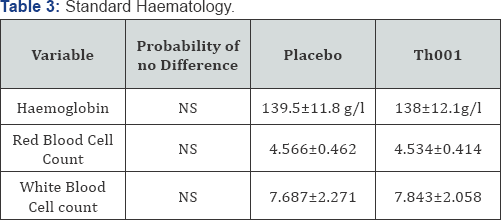
The statistically analysed groups of variables relevant to haemostasis are presented in (Table 1). There was a statistically significant increase in platelet count in those patients receiving Th001 first. The effect on platelet count is more clearly shown by repeated measures analysis of variance in the patients given Th001 first (Figure 1). The effect of Th001 upon biochemical variables is presented in (Table 2). All comparisons made by Wilcoxon. There were small but statistically significant rises of LFTs in patients taking Th001. With few exceptions the changes were within the normal range. In patients whose LFTs were elevated in the control period, a significant further rise on Th001 was found only for gamma GT. The increase in LFTs would not have been detected but for the paired design; the increases were almost all small and within the normal range. This effect may not be detectable at a lower dose, eg 5mg od. Prothrombin fragments F1+2 were mostly in the normal range and showed a non-significant decrease in patients to whom Th001 was administered. The results of standard haematology tests are presented in (Table 3).

ECG and adverse events
There were no statistically significant differences in QT interval or corrected QT interval (QTc) in any of the electrocardiograms. Adverse events were scaled as 0 =denied, 1 =mild, 2 =moderate. 3 =severe. They were present in 17 patients at scale 1, the remainder at scale 0. The difference between adverse events in the placebo and Th001 periods was not statistically significant (p =0.59). No significant drug interactions were found. However one patient whose control heart rate was 48/min experienced a fall of heart rate to 37/min while taking Th001 and required reduction of his atenolol dose. Two patients taking bisoprolol had slight increases in heart rate. The remaining patients on β-adrenoreceptor antagonists experienced variable heart rate responses, which were significantly reduced in this sub-group. There was no significant change in heart rate in patients not taking β-adrenoreceptor antagonists.
Discussion
Efficacy of the inhibition of arterial thrombosis by serotonin 5HT2A antagonists is attested by numerous animals studies of the effect of 5HT2A antagonists [7-17]. Macroaggregate platelet- rich aggregate growth is inhibited by ICI 170809 in hirudinised human blood by single platelet counting [18].
The important aspects of the present study are (1) lack of risk of bleeding and (2) lack of adverse events, both indicating that Th001 is a safe drug for the treatment and prevention of platelet-rich arterial occlusive thrombosis, assuming that efficacy for that effect can be shown in man. The small effect on liver function (similar to that found in a larger cohort by ICI) would not have been found without the paired design, and may disappear when the dose is reduced to 5mg od from the 10mg bd used here. The choice of 10mg bd was fortunate in that the resulting plasma concentrations were far above any likely levels in future studies with lower doses; this reinforces the value of the safety data. An effective dose may be as low as 5mg od. Also, with lower doses, patients are less likely to experience β-adrenoreceptor antagonist induced bradycardia below 60/ min requiring the dose of β-adrenoreceptor antagonist to be reduced.
The dose of 10mg bd was adopted upon the advice of one of the co-authors of the paper by [9]. When the plasma samples were assayed after the end of the study, it was found that the values were approximately four times those that were maximally effective in the study by [1], in dogs. This miscalculation led us to conclude that it enhanced the value of the safety data as no significant adverse event were recorded with this excessive dose apart from the effect on liver function tests. It is a little surprising to see a persistence of the drug after 2 weeks at a dose of 10mg. The fact that this is seen in only some individuals points to variations in drug metabolism (e.g. by cyp polymorphisms), or excretion. Full details of these considerations are available in the ICI (now Thromboserin Ltd) archive.
The assessment of platelet function lacks specific analysis of pathways that might be affected by 5HT in vivo. 5HT is well known to augment the platelet response to other agonists, while not acting as a primary agonist at physiological concentrations. We saw very little response of the platelets to 5HT alone. Since the planning of the present study in 2000, there have been new advances in the study of platelet function, which obviously cannot be performed retrospectively. The effect of Th001 upon these more sensitive indices are planned for when funding is available for re-manufacture of the drug, that being required for regulatory reasons. Therefore, we did not design our study to reveal efficacy, and studied stable out-patients not undergoing active thrombosis. We attempted to settle doubts about any effect on haemostasis of our chosen 5HT2A antagonist [1], formerly ICI 170809 for treatment of mental disorders, but patented for use as an anti-thrombotic drug by Thromboserin Ltd in December 2015. Th001 passed the haemostasis tests, there being no effect on skin bleeding time and flow cytometric tests of haemostasis. Adminstration of ICI 170809 to animals undergoing thoracotomy by McAuliffe et al. [1] did not induce any bleeding from the wounds in which blood vessels had been subjected to diathermy burns.
We hesitate to interpret the increase in platelet count in patients receiving Th001 in the first treatment period. Our speculation that this could be accounted for as a reduction in platelet consumption would need to be tested and is best dismissed for the time being as an speculation influenced by bias (see conflict of interest). The lack of bleeding risk indicates that Th001 would be a potential safe therapy (if efficacy can be proven in patients) for the prevention of arterial thrombotic occlusion around the time of surgical operations, when other anti-thrombotic treatments are contra-indicated because of surgical bleeding. Our patients were stable, and therefore had little, if any growing thrombi at arterial stenoses. Such growing thrombi are characteristic of acute thrombus related clinical conditions in arterial disease.
Conclusion
We conclude that the absence of any pro-haemorraghic effect of Th001, and the excellent safety profile thus far, may make this a most suitable anti-thrombotic agent for further development.
Acknowledgement
The senior pharmacologist supervising this study, Professor Gabrielle Hawksworth, is now deceased. We dedicated this write-up to her memory. Many thanks are due to clinical staff in the cardiology research room, and technicians in the University laboratories, for their work and support. The funding consisted of academic grants to the University of Aberdeen from the Coronary Thrombosis Trust, Enterprise Scotland Grampian and Tenovus Scotland.
Conflicts of Interest
There were no conflicts ofinterest at the time of the study (2006-2007) but subsequently Noble and Drake-Holland became share holders in Thromboserin Ltd who hold the patents of use for Th001, formerly ICI 170809, the drug used in this academic study.
For more Open Access Journals in Juniper Publishers please
click on: https://juniperpublishers.com
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php
For more Open
Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment