Pro-Atherogenic Oxidized Ldl/β2-Glycoprotein I Complexes in Diabetes Mellitus: Antioxidant Effect of Statins-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY &
CARDIOVASCULAR THERAPY
Abstract
Premature atherosclerotic cardiovascular disease
(CVD) is a well known complication of diabetes mellitus (DM) associated
with significant morbidity and mortality. The development of
atherosclerosis is largely promoted by oxidative stress and chronic
inflammation. Elevated low-density lipoprotein (LDL) is a known
atherosclerotic risk factor but LDL must be modified to become
atherogenic. Inflammatory-derived reactive oxygen and nitrogen species
oxidize LDL (oxLDL) giving rise to lipid peroxides and aldehydes that
favor the initiation and progression of atherosclerotic lesions.
Beta-2-glycoprotein I (β2GPI) is a lipid binding plasma protein with
pleiotropic functions that binds oxLDL via specific oxidative-derived
ligands to form pro-atherogenic oxLDL/β2GPI complexes and in this guise
exerts a buffering effect upon LDL oxidation. Statin (Rosuvastatin)
treatment lowered serum levels of oxLDL/β2GPI complexes in a group of DM
patients compared to statin untreated DM patient. The oxLDL/β2GPI
decrease was independent from the reduction of cholesterol, LDL and
triglycerides but likely dependent on Rosuvastatin reduction of nitrates
(NO3-) suggesting that Rosuvastatin may impact on the oxidative
metabolism of lipids and/or LDL. In addition, the oxLDL/β2GPI complex
may represent a surrogate marker of oxidative inflammation in DM.
Introduction
Diabetes mellitus (DM) is the fifth deadliest disease
in the United States with an annual economic cost estimated over $100
billion. Cardiovascular disease (CVD) represents the most life
threatening consequence of DM accounting for the death of up to 65% of
DM patients. Aggressive efforts aimed at treating and controlling the
classic CVD risk factors over the last few decades have brought along a
marked reduction in CVD morbidity and mortality in the US, though the
morbidity and mortality attributable to CVD from DM and obesity
continues to show an upward trend [1,2].
The laboratory diagnosis of DM relies on the presence
of abnormal fasting glucose and/or an abnormal glucose tolerance test
alongside abnormalities of lipid and protein metabolism due to defects
in insulin production or activity [3]. All these metabolic abnormalities
lead to a pro-atherogenicoxidative inflammatory environment. Recent
research has further unraveled the pathogenic mechanisms of CVD in DM
mostly due to intrinsic rather than extrinsic factors. Because CVD
remains the main cause of death in DM, there is a strong need to
identify more specific mechanisms that can be acted upon to develop
better CVD prevention and bend down the incidence and mortality curves
[4].
Atherosclerosis is a chronic progressive disease
(Figure 1) characterized by two low grade inflammatory components, one
prevalently systemic that starts early in life affecting the vascular
endothelium, monocytes and platelets, and another localized to the
arterial wall (plaques) that develops in later adulthood [5,6]. Early
identification and intervention is important to prevent disease
progression. The complex inflammatory process initiates as oxidative
stress (lipoprotein oxidation) and progresses with the participation of
immuno-inflammatory mononuclear cells of the innate and adaptive
immune system [7,8]. The newly issued American College
of Cardiology and American Heart Association (ACC/AHA-
2103) Guideline on the Treatment of Blood Cholesterol to
Reduce Atherosclerotic Cardiovascular Risk in Adults took
these concepts into consideration by diverting the focus away
from just measuring cholesterol into taking in consideration
LDL, statin response and inflammatory biomarkers as more
clinically relevant risk factors [9].

Oxidative stress and low grade chronic inflammation
(oxidative inflammation) contribute to premature
atherosclerotic CVD in DM [10]. Indeed, the abnormal lipid
profile of diabetes associates biochemically with lipid
peroxidation, a process whereby superoxide radical (O2•-
) released by neutrophils or endothelial cells may attack
double bonds of arachidonic acid allowing the formation of
oxygen containing cyclic structures termed isoprostanes
[11]. Isoprostanes are recognized markers of in vivo oxidative
stress and their plasma or urinary concentrations are elevated
in DM [12,13]. In the course of oxidative inflammation,
endothelial and mononuclear cells also generate additional
reactive nitrogen species (RNS) including nitric oxide (NO•)
that behaves as a pathogenic mediator and/or as a cytotoxic
molecule [14]. However, most of NO• mediated pathogenicity
depends on the formation of secondary intermediates such as
peroxynitrite anion (ONOO-) and nitrogen dioxide (•NO2) that
are more reactive and toxic than NO• [15]. In the presence of
superoxide radical (O2•-), NO• gives rise to ONOO-, a strong
highly reactive oxidant with very short biological half-life
producing nitrated proteins [16].
Reactive oxygen species (ROS) and RNS may exert free
radical attack on low-density lipoproteins (LDL) releasing lipid
peroxides and highly reactive aldehydes (4-hydroxynonenal)
that form specific adducts with lysine inducing the posttranslational
modification of lipoproteins, with consequent
gain or loss of function. During the same process LDL becomes
oxidized (oxLDL) turning into a highly pro-inflammatory and
atherogenic [17,18]. Beta2-Glycoprotein I (β2GPI) is a lipidbinding
plasma protein involved in thrombosis, fibrinolysis,
apoptosis, atherosclerosis and angiogenesis [19]; it binds
oxLDL via specific oxidative-derived ligands to form oxLDL/
β2GPI complexes [20]. Elevated plasma levels of oxLDL/
β2GPI complexes were initially described in patients with
antiphospholipid syndrome (APS) [21] and systemic lupus
erythematosus (SLE) [22], but later found in non-autoimmune
chronic inflammatory diseases such as chronic nephropathies,
coronary artery disease, myocardial infarction and DM
[23,24]. OxLDL and β2GPI have been co-localized in human
atherosclerotic lesions by immune-hysto chemical staining
implying a pro-atherogenic role [25,26]. In the presence
of anti-β2GPI antibodies, macrophages ingest oxLDL/β2GPI
complexes at an enhanced rate providing further support
for their pro-atherogenic role [27,28]. Current experimental
evidence, including in vivo imaging techniques, identified
the atherosclerotic lesion as the primary site of oxLDL/β2GPI
complex formation [20,29].
In DM, serum levels of oxLDL/β2GPI complexes were
particularly elevated in patients with greater intima-media
thickness (IMT) [30], but were lower in patients taking statins
[24]. These observations indicate that oxLDL/β2GPI complexes
may behave as modifiable biomarkers and/or as risk factors
for atherothrombotic complications of DM. In addition, the
lower oxLDL/β2GPI concentration in DM patients on statins
suggested that this class of drugs may prevent or decrease
the oxidative modification of LDL possibly by an antioxidant
mechanism. Indeed HMG-CoA reductase inhibitors (statins)
bear antioxidant properties in addition to their lipid-lowering,
anti-thrombotic and anti-inflammatory effects [31,32]. We
tested the hypothesis that Rosuvastatin had antioxidant
effects in DM by performing an open label interventional
trial and observed a significant change in serum oxLDL/β2GPI
concentration as the primary endpoint. In this review we
discuss the role of oxidative stress in atherogenesis and the
antioxidant effect of statins on oxLDL/β2GPI complexes.
The pathogenesis of atherosclerosis in DM is multi factorial:
- Chronic hyperglycemia from insulin deficiency [33,34].
- Chronic dyslipidemia characterized by decreased high-density lipoprotein (HDL), changes in the HDL subpopulations, raised triglycerides, and unchanged or only slightly elevated low-density lipoprotein (LDL) [35].
- Metabolic syndrome characterized by obesity, dyslipidemia, hypertension and insulin resistance [36]. All three promote increased oxidative stress that initiate and perpetuate vascular damage and atherothrombotic complications [37].
Under physiologic conditions, oxidation should be well
counteracted by natural enzymatic and non-enzymatic
antioxidant mechanisms. In DM, oxidation overrides
antioxidant mechanisms [38,39] and initiates endothelial
dysfunction by favoring the expression of a pro-adhesive and
pro-thrombotic surface that allow the migration of immunoinflammatory
cells into the arterial wall (Figure 1). There, local
pro-chemotactic and inflammatory cytokines further recruit
and activate immuno-inflammatory cells that propagate lipid
accumulation, oxidative inflammation and the development
of the typical progressive atherosclerotic lesions (plaques)
[40,41]. Moreover, early inflammation increases the expression
of cell surface receptors and the intracellular accumulation of
oxLDL by local arterial mononuclear cells process mediated by
scavenger and Fcγ receptors [28].
Multiple efforts by several groups aimed at enhancing the
antioxidant defense in DM and CVD patients. Serum and urine
bio makers of systemic oxidative stress correlated with blood
glucose levels and responded to anti-diabetic intervention[42,43]. In vivo s tudies i ndicated t hat o xidative s tress f rom
hyperglycemia starts well before clinical complications
become evident, underscoring the importance of glucose
control to minimize long term complications of oxidative
inflammation in DM. Metformin treatment lowered urinary
excretion of 8-isoPGF2a and 11dhTxB2 in newly diagnosed
DM patients suggesting that despite a good metabolic
improvement, metformin also behaved as an antioxidant and
antithrombotic agent in DM [44]. Some epidemiological studies
have demonstrated a weak inverse relationship between
stroke risk and ingestion of antioxidant foods. Other clinical
trials have shown conflictive results regarding the protective
effect of antioxidants against CVD outcomes [45,46]. Several
ongoing clinical trials are assessing the effectiveness of statins
from an antioxidant perspective; so far these studies have
suggested a close relationship between oxidative inflammation
and atherogenesis but the usefulness of antioxidant-based
therapeutics on CVD remains controversial.
Oxidation of LDL is a key contributor to the initiation and
progression of atherosclerosis [7,47] and is a complex process,
in going from “minimally oxidized” to more “extensively
oxidized” LDL particles induces the expression of adhesion
molecules on endothelial cells and the release of chemotactic
cytokines into the circulation [48]. These events allow blood
monocytes to adhere to the arterial wall and to migrate into
the arterial intima, where they differentiate into macrophages.
In turn, these activated macrophages enhance a pro-oxidant
environment of the arterial wall, causing intensive oxidative
modification of LDL lipoproteins including cholesteryl esters,
phospholipids and apolipoprotein B [49]. Because oxLDL
becomes unrecognizable by LDL receptors, it is taken up by
scavenger receptors, which facilitate a persistent intracellular
accumulation of LDL by macrophages [50] transforming
them into the characteristic foam cells. As the lesion evolves,
these elements contribute to the morphological changes that
characterize the vulnerable plaques with an unstable lipidrich
necrotic core. Advanced lesions may undergo a necrotic
breakdown and plaque rupture that precipitate intra-vascular
thrombosis with acute occlusion clinically expressed as
unstable angina, myocardial infarction, stroke, and/or sudden
cardiac death [51].
A lthough t he o xidation o f L DL o ccurs p rimarily i n t he
vascular wall, recent studies have provided evidence for the
presence of oxLDL in blood [52]. Indeed numerous studies have
established oxLDL as an effective marker for the presence of
atherosclerosis, detecting both subclinical disease and more
advanced or severe CAD [53,54]. Because oxLDL is highly
unstable with a very short half-life (30 seconds) in the systemic
circulation [55], it is difficult to measure accurately by common
immunoassays. In addition, some lipid binding plasma proteins
such as β2GPI interact with circulating oxidized lipoproteins tobuffer their deleterious effects. This may cause reduced assay
sensitivity and false-negative results as most of the oxLDL
assays use monoclonal antibodies directed against just one or
a few of the epitopes present on lipid or protein moieties. This
phenomenon has hampered the use of oxLDL in CVD clinical
trials and clinical laboratory to assess its predictive role in
atherogenesis.
Because immune-staining of human atherosclerotic lesions
co-localized β2GPI with oxLDL, the relationship between these
molecules was further investigated [25,26]. β2GPI is a 50-kDa
single-chain phospholipid-binding plasma protein composed
of 326 amino acid residues arranged in 5 homologous repeats
or domains. The fifth domain contains a positively charged
amino acid patch important in anionic phospholipid and oxLDL
binding [56]. Unlike native LDL, β2GPI binds oxLDL via specific
oxidative-derived ligands to form stable and pro-atherogenic
oxLDL/β2GPI complexes [20,57] in an attempt what to
quench in an antioxidant fashion the pro-inflammatory and
pro-atherogenic effects of oxLDL. But in doing so, oxLDL/
β2GPI complexes also become immunogenic triggering the
production of pro-atherothrombotic auto antibodies and
immune complexes.
It is now recognized that the immune system plays a role
in blood coagulation. Autoimmune-mediated thrombosis
refers to auto antibodies that promote venous and arterial
thromboembolic events in patients with systemic lupus
erythematosus and antiphospholipid syndrome who develop
premature atherothrombotic CVD with significant morbidity
and mortality [58,59]. Endogenous pro-atherogenc oxLDL/
β2GPI complexes initially described in autoimmunity [22]
have been associated with the development of atherosclerotic
CVD in non-autoimmune diseases [23,24]. Serum levels
in higher oxLDL/β2GPI quartiles were associated with an
geographically determined disease severity and give a 3.5
risk for adverse outcomes in acute coronary syndromes
[60,61]. Interestingly, statin treatment reduced oxLDL/β2GPI
complexes independently from LDL-lowering effects likely via an antioxidant mechanisms [62,63]. Thus, oxLDL/β2GPI
complexes meet current criteria for biomarkers of CVD risk:
- To have a direct mechanistic relevance to atherosclerosis (causal relationship).
- To be measured quantitatively with available technology that is accurate, reproducible and cost effective.
- To permit patient stratification for severity and outcomes.
OxLDL/β2GPI and its immune complexes up-regulate
the macrophage expression of scavenger and Fcγ receptors,
favoring enhanced oxLDL/β2GPI uptake followed by its rapid
accumulation in lysosomes where an immune response (innate
and adaptive) may be mounted. Experiments evaluating
the intracellular trafficking of β2GPI within macrophages
showed that free β2GPI was poorly incorporated in late
endosomes and stagnated there, whereas complexed β2GPI
(to phosphatidylserine liposomes or oxLDL) was quickly
transported to lysosomes; the addition of antibodies to β2GPI
further accelerated this process [64]. β2GPI auto reactive
CD4+ T cells have been identified in patients with APS that
preferentially recognized a cryptic peptide (residues 276-
290) in β2GPI domain V that contains the phospholipid-binding
site. Macrophages stimulated with phospholipid-bound β2GPI
induced an immune response to peptide 276-290 in a HLA-DRrestricted
manner, while β2GPI or phospholipids alone did not
[65]. In this respect, β2GPI can be viewed as a component of
the innate immunity; but once bound to oxLDL, the complex
may shift to the generation and maintenance of an adaptive
immune response that play an important role in atherogenic
inflammation via the inflammasome/IL-1B system [39].
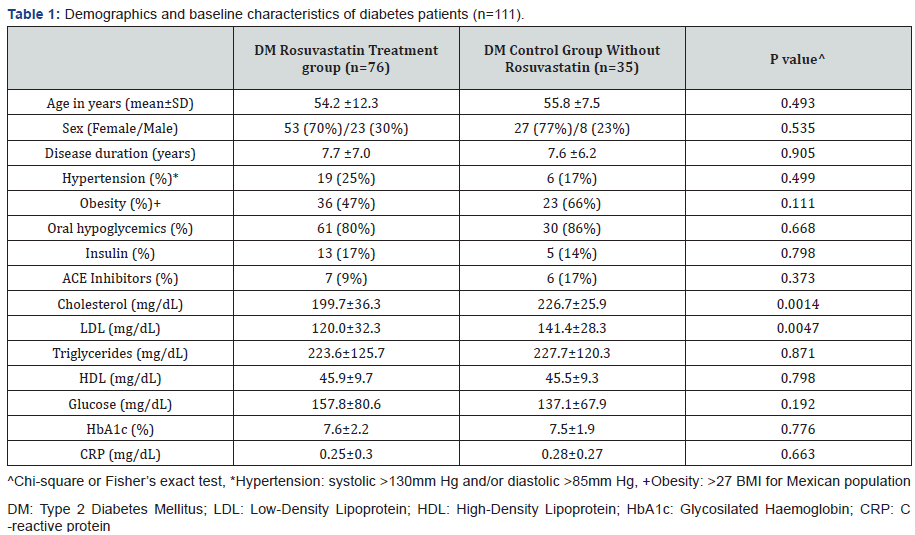
OxLDL/β2GPI complexes are indicative of systemic
oxidative inflammation in obese middle age men and DM, and
may be used to assess pro-atherogenic pathways because
circulating levels of oxLDL independently predict future CVD
events [24,66,67]. It was particularly important to determine
effective ways to modify oxLDL/β2GPI levels as these
complexes have been associated with the severity and adverse
outcomes of coronary disease [60,61]. The effect of statins on
oxLDL/β2GPI complexes was studied by our group [62] on 111
type 2 DM patients (80 females, 31 males, mean age of 54.7
years). One group of 76 patients received 10mg daily for 6
weeks of oral Rosuvastatin while a control group of 35 patients
did not receive Rosuvastatin. Serum samples taken at baseline
and after 6 weeks were tested at the end of the study. The
baseline clinical and laboratory variables of DM patients taking
Rosuvastatin and control groups are shown in Table 1. DM
patients in the Rosuvastatin group were stratified according
to their lipid profile. In addition to oxLDL/β2GPI complexes,
nitrite (NO2-), nitrate (NO3-), asymmetric dymethyl arginine
(ADMA) nitrotyrosine (NT) and paraoxonase activity (PON)
were measured in all samples.
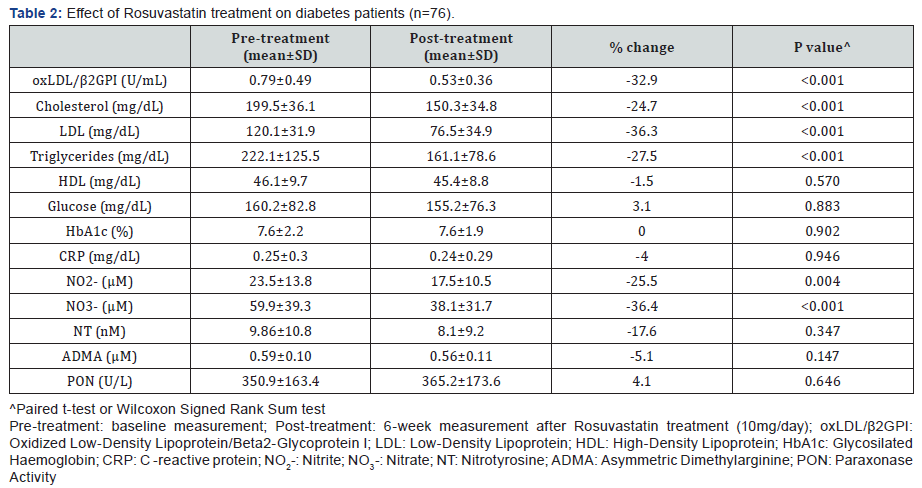
Rosuvastatin treatment caused a significant decrease
of oxLDL/β2GPI complexes (32.9%) along with cholesterol
(24.7%), LDL (36.3%) and triglycerides (27.5%). Among
the nitric oxide metabolites, Rosuvastatin treatment also
decreased NO2- (25.5%) and NO3- (36.4%) (Table 2). The
observed decrease of oxLDL/β2GPI complexes was more
noticeable in patients with dyslipidemia (37.4%) compared
to those with normal lipid profile (22.4%). Interestingly, NO2-
decreased more in dyslipidemics than in non-dyslipidemic
patients (29% vs 18.8%) while NO3- decreased in the same way
(42.9% vs 21.8%). The decrease of oxLDL/β2GPI complexes by
Rosuvastain t reatment in these DM patients was independent
of the lipid lowering effects of the statin. Further, only NO3-
was an independent predictor of oxLDL/β2GPI complexes
(t=2.0, p=0.04).
Support to an antioxidant effect of statin indirectly
assessed by a decrement of oxLDL/β2GPI complexes comes
from very few studies.
- A randomized, double blind, placebo controlled pilot study of 37 consecutive SLE patients receiving 40mg daily atorvastatin or placebo for 12 months demonstrated a decrease of oxLDL/β2GPI complexes [63]. In this study, after correction for age and disease duration oxLDL/β2GPI complexes decreased by 27% (p=0.002).
- Blinden et al. [68] studied the effect of statin therapy (Atorvastatin, Simvastatin, Rosuvastatin, Lovastatin, Pravastatin and Fluvastatin at doses between 5-80mg) in 186 coronary artery disease patients undergoing elective cardiac catheterization. There was a significant dosedependent reduction of oxLDL/β2GPI complexes, more noticeable at atorvastatin dose equivalents between 20- 80mg.
- Statin influence of oxLDL/β2GPI levels on CVD patients have been further confirmed by Berger et al. [69] and Gurbel et al. [70]. This effect was independent and inversely associated with inflammation. These finding support the concept of a dose dependent anti-oxidant effect of statins.
Our evaluation of the significance of oxLDL/β2GPI complexes
in DM demonstrated an independent association with some
clinical (obesity and hypertension) and biochemical variables
(nitric oxide metabolites) [62]. OxLDL/β2GPI complexes were
higher in males than females. This gender difference reflects
the notion that in DM oxidative inflammation is enhanced
[71] particularly in men [72]. With regards to biochemical
variables the only independent predictor of oxLDL/β2GPI was
nitrate (NO3-). This RNS may be viewed as an “inflammatory
metabolite” of NO• (as opposed to NO2- that may be viewed as
the “vascular” metabolite). Thus, NO3- may contribute to LDL
oxidation and formation of the oxLDL/β2GPI complex in DM
[20].
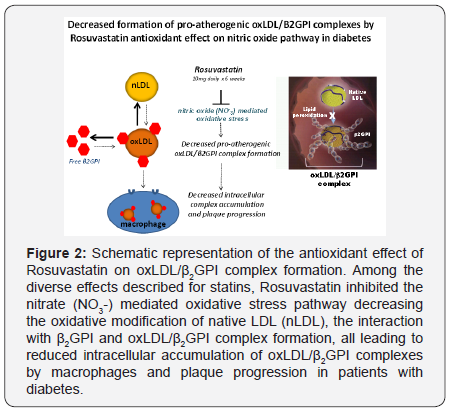
Rosuvastatin administered daily for 6 weeks caused
a significant reduction of serum oxLDL/β2GPI complexes
(Figure 2). This reduction was accompanied by lower total
cholesterol, LDL and tryglicerides, particularly in patients
with dislipidemia. However, the reduction of oxLDL/β2GPI was
statistically independent of any statin-mediated decrease of
total cholesterol, LDL and tryglicerides. It is important to point
out that oxLDL/β2GPI levels were higher in DM patients with
dyslipidemia, consistent with the concept that patients with
elevated lipid levels may be prone to or sustain more intense
oxidative damage.
Statins inhibit the enzyme HMG-CoA reductase, preventing
the generation of mavelonate and the subsequent biosynthesis
of cholesterol. Mevalonate is also a precursor of isoprenoid
intermediates and one of these geranylgeranylated proteins
(RhoA) is implicated in intracellular signaling [73,74]. Through
the inhibition of protein prenylation, such as Ras and Rho,
statins activate the MAPK cascade or NF-κB pathways that
induce proteins with anti-inflammatory, anti-proliferative
and anti-thrombotic effects [75]. In addition, by acting on
SREBP-2, statins up-regulate the expression of genes coding
for paraoxonase, the enzyme that accounts for most of the
antioxidant effect of HDL [76]. Thus, the inhibition of RhoA by
statins have a number effects on the vasculature that could be
beneficial in hypercoagulable disorders by improving nitric
oxide synthase activity, regulation of angiogenesis, reduction
of vascular inflammatory and prothrombotic activities and
atherosclerotic plaque stabilization [77,78]. By using plasma
biomarkers of oxidation such as oxLDL/β2GPI, we can clinically
evaluate the effect of treatment on this event.
Because the benefits of statins on the cardiovascular
system are beyond those on cholesterol metabolism we
speculated t hat Rosuvastatin may exert an antioxidant effect,
either by enhancing the activity of PON and of nitric oxide
synthase or by interfering with oxidative inflammatory
mechanisms that promoted the generation of oxLDL and their
consequent interaction with β2GPI [79-82].
Our studies suggest that statins would have the same
antioxidant effect on oxLDL/β2GPI complex formation in
patients with metabolic syndrome and obesity. Fatty liver
disease, particularly non-alcoholic steatohepatitis (NASH),
is not only associated with insulin resistance, obesity,
metabolic syndrome, liver fibrosis/cirrhosis, but also
with atherosclerotic CVD [83]. It has been proposed that
dyslipidemia, inflammation, oxidative stress and macrophage
activation are early events in NASH, similar to atherosclerosis
and perhaps they represent shared aspects of a similar disease
process [84]. In this case, stains may have a more prominent
therapeutic role as antioxidants are considered first line
treatment for NASH.
These studies demonstrate that treatment with
Rosuvastatin reduced serum levels of oxLDL/β2GPI in DMpatients. The implications of these findings are twofold:
statins independently reduce lipids and NO3- suggesting an
antioxidant effect possibly mediated via lipid/nitric oxidative
pathways; and that oxLDL/β2GPI complexes may be viewed as
serologic biomarkers of oxidative stress.
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment