Lipids and its Metabolism-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
The main biological function of the lipids includes
storing energy, signaling, and acting as structural components of cell
membrane. Lipids, such as cholesterol and triglycerides, are insoluble
in plasma and circulating lipids are carried in lipoproteins to various
tissues for energy utilization, lipid deposition, steroid hormone
production, and bile acid formation. This review article will present
the different types, functions and metabolism of the lipids.
Cholesterol
Cholesterol is designated as 3-betahydroxy-5,
6-cholestene. Cholesterol is widely distributed in all the cells of the
body, particularly abundant in nervous tissues. Higher concentration of
cholesterol is found in brain, liver, kidney, adipose tissue and
suprarenal glands in both free and esterified forms. In plasma,
esterified form comprises 65-75% and in tissues, free form dominates
[1]. Liver appears to be chief source of endogenous cholesterol but it
is also synthesized in adrenals, intestinal mucosa and brain. Dietary
sources of cholesterol are mainly from food of animal origin.
Greater part of cholesterol of the body arises by
synthesis (about 1gm/day), whereas 0.3gm/day is provided by the average
diet. Virtually all tissues containing nucleated cells are capable of
synthesizing cholesterol, particularly the liver, adrenal cortex, skin,
intestine, testes, etc. In humans, the total plasma cholesterol is about
200mg/dl. The greater part is found in esterified form [2,3]. It is
transported as lipoproteins in plasma. Higher proportion of it is found
in LDL, to some extent in VLDL and HDL. Cholesterol in diet is absorbed
from the intestine. Along with other lipids, including the cholesterol
synthesized in the intestine are incorporated in to Chylomicrons and
VLDL.
Approximately half of the cholesterol eliminated from
the body is excreted in the faeces after conversion into bile salts.
The remainder is excreted as neutral steroids. Much of the cholesterol
secreted in the bile is reabsorbed. It is believed that the cholesterol
that serves as precursors for the faecal sterol is derived from the
intestinal mucosa [4]. A large portion of biliary excretion of bile
salts is reabsorbed in to the portal circulation, taken up by the liver
and re-secreted in the bile. This is known as entero hepatic
circulation. The bile salts, which are not reabsorbed, are excreted in
faeces.
Serum cholesterol levels have been given special
attention as it gives direct evidence of various metabolic disorders and
development of atherosclerosis. Elevated cholesterol concentration is
considered prime risk factor for coronary heart disease. The Framingham
study shows linear increase in coronary risk with increment in total
plasma cholesterol concentration from 180mg/dl upwards.
They form the major bulk of the diet and major
fraction of natural fats. Triglycerides are formed by combination of one
glycerol molecule with 3 molecules of fatty acids, which may be same or
different. The chief site of synthesis of triglycerides is liver and
adipose tissue via glycerol phosphate pathway [5]. In plasma they are
contained in VLDL.
The ingested triglycerides are converted in to low
grade triglycerides, free fatty acids and mono glycerides by pancreatic
lipase. These fatty acids and mono glycerides pass in to intestinal
mucosal cells and are re-esterified to triglycerides, which are then
incorporated with lipoproteins and carried in blood, contained in
chylomicrons. These
chylomicrons and VLDL are cleared from plasma by the action
of lipoprotein lipases, forming FFA and LDL [6]. The FFA binds
loosely with plasma albumin and has high turnover rate (halflife
2-3 minute). Quite a few amino acids may yield acetyl CoA,
which may contribute to triglyceride synthesis. Hence excess
of protein is stored as triglycerides.
The lipoprotein consists of esterified and unesterified
cholesterol, triglycerides, phospholipids, and protein. Based
on the physicochemical characteristics of lipoproteins, these
particles have been classified by their lipoprotein subclass size
and concentrations [7]. There are five major lipoproteins, each
of which has a different function.
Chylomicrons are very large particles that carry dietary
lipid. They are associated with a variety of apolipoproteins,
including A-I, A-II, A-IV, B-48, C-I, C-II, C-III, and E.
Very low density lipoprotein (VLDL) carries endogenous
triglycerides and to a lesser degree cholesterol. The major
apolipoproteins associated with VLDL are B-100, C-I, C-II, C-III,
and E.
Intermediate density lipoprotein (IDL) carries cholesterol
esters and triglycerides. It is associated with apolipoproteins
B-100, C-III, and E.
Low density lipoprotein (LDL) carries cholesterol esters
and is associated with apolipoproteins B-100 and C-III.
High density lipoprotein (HDL) also carries cholesterol
esters. It is associated with apolipoproteins A-I, A-II, C-I, C-II,
C-III, D, and E.
Understanding the major functions of the different
apolipoproteins is important clinically, because defects in
apolipoprotein metabolism lead to abnormalities in lipid
handling [8].
The assembly and secretion of apolipoprotein B containing
lipoproteins in the liver and intestines is dependent upon
microsomal triglyceride transfer protein, which transfers
lipids to apolipoprotein B. In one study, apolipoprotein B and
microsomal transfer protein genes were expressed in the
human heart, strongly suggesting that the heart synthesizes
and secretes apolipoprotein B containing lipoproteins [9]. Thismay represent a pathway of «reverse triglyceride transport»
by which the cardiac myocytes can unload surplus fatty acids
not required for fuel.
- A-I-Structural protein for HDL; activator of lecithincholesterol acyltransferase (LCAT).
- A-II-Structural protein for HDL; activator of hepatic lipase.
- A-IV-Activator of lipoprotein lipase (LPL) and LCAT.
- B-100-Structural protein for VLDL, IDL, LDL, and Lp (a); ligand for the LDL receptor; required for assembly and secretion of VLDL.
- B-48-Contains 48 percent of B-100; required for assembly and secretion of chylomicrons; does not bind to LDL receptor.
- C-I-Activator of LCAT.
- C-II-Essential cofactor for LPL.
- C-III-Interferes with apo-E mediated clearance of triglyceride-enriched lipoproteins and remnants by cellular receptors, particularly in the liver [10] inhibits triglyceride hydrolysis by lipoprotein lipase and hepatic lipase [11] has multiple proatherogenic effects on the arterial wall, including interfering with normal endothelial function [12,13].
- D-May be a cofactor for cholesteryl ester transfer protein.
- E-Ligand for hepatic chylomicron and VLDL remnant receptor, leading to clearance of these lipoproteins from the circulation ligand for LDL receptor. There are three different apo E alleles in humans: E2, which has cysteine residues at positions 112 and 158; E3, which occurs in 60 to 80 percent of Caucasians and has cysteine at position 112 and arginine at position 158; and E4, which has arginine residues at positions 112 and 158 [14]. These alleles encode for a combination of apo E isoforms that are inherited in a codominant fashion. Compared to apo E3, apo E2 has reduced affinity and apo E4 has enhanced affinity for the LDL (apo B/E) receptor. These isoforms are important clinically because apo E2 is associated with familial dysbetalipoproteinemia (due to less efficient clearance of VLDL and chylomicrons) and apo E4 is associated with an increased risk of hypercholesterolemia and coronary heart disease.
- Apo (a) Structural protein for Lp (a); inhibitor of plasminogen activation on Lp (a).
Lipoprotein metabolism can be divided into exogenous and
endogenous pathways. The exogenous pathway starts with the
intestinal absorption of dietary cholesterol and fatty acids.The mechanisms regulating the amount of dietary cholesterol
that is absorbed are unknown (Figure 1).
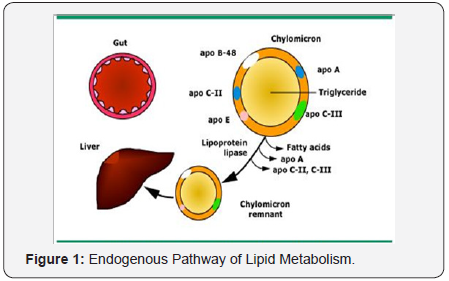
Within the intestinal cell, free fatty acids combine with
glycerol to form triglycerides, and cholesterol is esterified by
acyl-coenzyme A: cholesterol acyltransferase (ACAT) to form
cholesterol esters. The important role of ACAT was established
in an animal model of ACAT deficiency, which found complete
resistance to diet-induced hypercholesterolemia due to lack
of cholesterol ester synthesis and reduced capacity to absorb
cholesterol [15]. Triglycerides and cholesterol are assembled
intracellularly as chylomicrons. The main apolipoprotein is
B-48, but C-II and E are acquired as the chylomicrons enter the
circulation. Apo B-48 permits lipid binding to the chylomicron
but not does not bind to the low density lipoprotein receptor,
thereby preventing premature clearance of chylomicrons from
the circulation before they are acted upon by lipoprotein lipase
(LPL).
Apo C-II is a cofactor for LPL that makes the chylomicrons
progressively smaller, primarily by hydrolysing the core
triglycerides and releasing free fatty acids. The free fatty acids
are then used as an energy source, converted to triglyceride,
or stored in adipose tissue. The end-products of chylomicron
metabolism are chylomicron remnants that are cleared from
the circulation by hepatic chylomicron remnant receptors
for which apo E is a high-affinity ligand. The chylomicron
remnants contain a smaller core of lipids that is enveloped by
excess surface components. These surface constituents are
transferred from the chylomicron remnant for the formation
of high density lipoprotein.
The endogenous pathway of lipid metabolism begins with
the synthesis of very low lipoprotein (VLDL) by the liver
(Figure 2). VLDL particles contain a core of triglycerides
(60 percent by mass) and cholesterol esters (20 percent by
mass). Microsomal triglyceride transfer protein (MTP) is an
intracellular lipid-transfer protein found in the endoplasmic
reticulum. It is essential for the transfer of the lipid molecules
(principally triglycerides) onto apolipoprotein (apo) B 100 in
the liver [16,17]. The surface apo lipo proteins for VLDL apo
C-II acts as a cofactor for lipoprotein lipase, apo C-III whichinhibits this enzyme, and apo B-100 and E which serve as
ligands for the apo lipo protein B/E (low density lipoprotein
[LDL]) receptor [8]. In the absence of functional MTP, VLDL
is not secreted into the circulation. The triglyceride core of
nascent VLDL particles is hydrolysed by lipoprotein lipase.
During lipolysis, the core of the VLDL particle is reduced,
generating VLDL remnant particles (also called intermediate
density lipoprotein [IDL]) that are depleted of triglycerides via
a process similar to the generation of chylo micron remnants.
Some of the excess surface components in the remnant particle,
including phospholipid, unesterified cholesterol, and apo lipo
proteins A, C and E, are transferred to high density lipoprotein
(HDL).
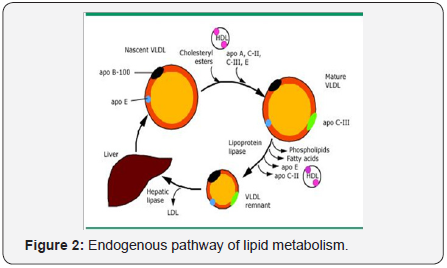
VLDL remnants can either be cleared from the circulation
by the apo B/E (LDL) or the remnant receptors or remodelled
by hepatic lipase to form LDL particles. There are four common
sequence polymorphisms in the hepatic lipase gene promoter;
the most frequent is a C to T substitution [18]. The presence
of a C allele is associated with higher hepatic lipase activity;
smaller, denser, and more atherogenic LDL particles, and
inversely with lower levels of HDL cholesterol [19].
LDL particles contain a core of cholesterol esters, lesser
amounts of triglyceride, and are enriched in apolipoprotein
B-100, which is the ligand for binding to the apo B/E (LDL)
receptor. LDL can be internalized by hepatic and non-hepatic
tissues. Hepatic LDL cholesterol can be converted to bile
acids and secreted into the intestinal lumen. LDL cholesterol
internalized by non-hepatic tissues can be used for hormone
production, cell membrane synthesis, or stored in the
esterified form.
The internalization of LDL is regulated by cellular
cholesterol requirements via negative feedback control of
apo B/E (LDL) receptor expression [20]. Cells in positive
cholesterol balance, for example, suppress apo B/E (LDL)
receptor expression. On the other hand, decreased activity of
HMG CoA reductase, the enzyme that controls the rate of de
novo cholesterol synthesis by the cell, leads sequentially to a
fall in cell cholesterol, increased expression of apo B/E (LDL) receptors, enhanced uptake of cholesterol from the circulation,
and a reduction in the plasma cholesterol concentration.
Chemically-modified LDL such as oxidized LDL can also
enter macrophages and some other tissues through the
unregulated scavenger receptor. This pathway can result
in excess accumulation of intracellular cholesterol and the
formation of foam cells, which contribute to the formation of
atheromatous plaques.
The importance of the LDL receptor in the regulation
of cholesterol metabolism has been demonstrated in both
experimental animals and humans. Knockout of the LDL
receptor in transgenic mice leads to a substantial elevation
in total cholesterol levels; a defect that can be reversed by
restoring the LDL receptor gene [21]. In humans, familial
hypercholesterolemia is often associated with a defect in the
LDL receptor [22].
Formation and metabolism of HDL involves the following
steps (Figure 3) [8,23]:

- Hepatic and intestinal synthesis of small nascent HDL particles composed of phospholipid and apolipoproteins.
- Then, there is procurement of surface components (phospholipids, cholesterol and apolipoproteins) from triglyceride-depleted chylomicron and VLDL remnants.
- Acquisition of free cholesterol from tissue sites and other lipoproteins, as the initial HDL particles contain relatively little cholesterol.
- Apolipoprotein A-I on the surface of HDL plays a central role in this process. It serves as a signal transduction protein to mobilize cholesterol esters from intracellular pools. After diffusion of free (unesterified) cholesterol onto HDL, the cholesterol is esterified to cholesterol esters by lecithincholesterol acyltransferase (LCAT), a plasma enzyme that is activated primarily by apolipoprotein A-I. By a similar mechanism, HDL can act as an acceptor for cholesterol released during lipolysis of triglyceride-containing lipoproteins.
Cholesterol efflux regulatory protein also appears to play
an important role in the uptake of cellular cholesterol by
HDL by promoting the transfer of intracellular cholesterol to
the cell membrane [24,25]. Mutations in the gene encoding
for this protein, ABC1, are associated with low serum HDL
concentrations in familial HDL deficiency and Tangier disease.
Lipid transfer proteins, such as cholesteryl ester transfer
protein, facilitate movement of these newly synthesized
cholesterol esters to apolipoprotein B-containing lipoproteins
(VLDL, IDL, and LDL). The cholesterol can then be delivered
to the tissues for steroid synthesis or storage. The net effect
of the last two steps is the removal of excess cholesterol from
cells, which constitutes most of the anti-atherogenic effect of
HDL.
Lipoprotein (a) or Lp (a) is a specialized form of LDL that
is assembled extra cellularly from Apo lipoprotein (a) and
LDL. Apo (a) linked to apolipoprotein B-100 on the surface
of LDL by disulfide bridges. The formation of apo (a): apo B
complexes require an LDL particle of a certain morphology and
composition. The structural integrity of LDL, and therefore Lp
(a) formation, are modulated by LCAT [26]. The apo (a) chain
contains five domains known as kringles [27]. The fourth
kringle contains regions that are homologous with the fibrinbinding
domains of plasminogen. Through this structural
similarity to plasminogen, Lp (a) interferes with fibrinolysis
by competing with plasminogen binding to plasminogen
receptors, fibrinogen, and fibrin.
The net effect is impaired plasminogen activation and
plasmin generation at the thrombus surface, leading to
decreased thrombolysis [28,29]. Lp (a) can also bind to
macrophages via a high-affinity receptor, possibly promoting
foam cell formation and localization of Lp (a) at atherosclerotic
plaques [30].
Most of the plasma VLDL are hepatic in origin and are
vehicles of triglyceride transport from the liver to extra hepatic
tissues. Ribosomes on rough endoplasmic reticulum of liver
cell synthesize Apo protein B-100. Lipids are incorporated with
B-100 lipoprotein to form VLDL. It is secreted by the hepatic
cells through fenestrated sinusoidal epithelium in to the space
of Disse and enters the blood stream. These nascent VLDLs,
once in circulation take up apo-protein C and apo-protein E
from HDL, which they are devoid of.
Liver does not catabolise nascent VLDL. The lipoprotein
lipase hydrolyses VLDL through mono and di-acyl glycerol tofree fatty acids and glycerol. Then, free fatty acids are taken
up by the tissues. Lipoprotein lipase requires phospholipids
and apo-protein C as cofactors, present on VLDL. Reaction
with lipoprotein lipase results in loss of 90% of Triglycerol,
apo-protein A and apo-protein C. The percentage amount of
cholesterol and its esters almost doubles. Apo-protein E is
retained by remnants.
A standard serum lipid profile measures the concentration
of total and HDL-cholesterol (HDL-C) as well as the
triglycerides. With these values, the LDL cholesterol (LDL-C)
concentration can be estimated.
Serum total and HDL-C are measured directly and can
be obtained in fasting or non-fasting individuals; there are
only small, clinically insignificant differences in these values
between measurements in the fasting or non-fasting state [31].
The total cholesterol can vary by 4 to 11 percent within
an individual due to multiple factors including stress, minor
illness, and posture [32]. Values may also vary between
different laboratories, with data suggesting that a single
measurement of serum cholesterol can vary as much as 14
percent [32,33]. Thus, in an individual with a «true» serum
cholesterol concentration of 200mg/dL (5.2mmol/L), the range
of expected values is 172 to 228mg/dL (4.5 to 5.9mmol/L)
[33,34]. These observations suggest that more than one
measurement of total cholesterol should be obtained when
treatment considerations demand a precise determination.
Serum HDL-C may demonstrate even greater variability [35].
Friedewald equation: LDL-C reported in the lipid profile is
generally calculated using the Friedewald formula, which
states:
LDL-C = Total cholesterol - VLDL-cholesterol (VLDL-C) -
HDL-C [36].
The total cholesterol in plasma or serum is the sum
of cholesterol found in each of the VLDL, HDL, and LDL
lipoprotein particle types. The Friedewald formula is applied
to lipid values measured in the fasted state. In a non-fasting
patient, the contribution of post-prandial chylomicrons to the
total lipoprotein pool makes the formula much less accurate.
VLDL associated cholesterol is approximated by dividing
the measured total triglyceride level by 5. With the measured
total and HDL-C and triglyceride, an approximate LDL-C can be
calculated. There are, however, several sources of error involved
in the estimation of LDL-C using the Friedewald formula. The
formula is valid only if the total triglyceride concentration is
less than 400 mg/dL (4.516mmol/L). In patients with more pronounced hypertriglyceridemia, LDL-C levels must be
measured directly (direct LDL), by ultra centrifugal single spin
analysis or immune precipitation technique.
The estimated LDL-C concentration is also influenced
by the method error from each of the independent lipid
measurements (total cholesterol, triglycerides, and HDLcholesterol).
The calculation of VLDL-C (from triglycerides)
underestimates the cholesterol content of the atherogenic,
intermediate density lipoprotein (IDL), and VLDL remnants.
The estimated LDL-C concentration includes cholesterol
contained in other lipoproteins, such as lipoprotein(a) and
lipoprotein-X.
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php
To know more about Juniper Publishers please
click on: https://juniperpublishers.business.site/



Comments
Post a Comment