Left Atrial Myxoma as a Rare Cause of Cardiogenic Shock in Octagerians: Report of a Case and Review of the Literature-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Introduction: Cardiogenic Shock (CS)
is a state of end-organ hypo perfusion due to cardiac failure. It
occurs in 5% to 8% of patients hospitalized with ST- elevation
myocardial infarction (STEMI). Although myocardial infarction with left
ventricle failure remains the most common cause of CS, it is crucial to
exclude or / and identify rare pathologies that mimic this condition.
Left atrial myxoma is the most common benign primary tumor of the heart,
accounting for up to 50% of primary cardiac tumors. CS or even sudden
cardiac death due to myxomas may result from either complete obstruction
of the mitral valve orifice or MI resulting from coronary artery
emboli.
Case Presentation: We report a
patient admitted to our department because of syncopal episode and
altered mental status due to a large left atrium myxoma. The patient was
treated surgically, the myxoma was removed and the systemic
manifestations of organ malperfusion were reversed.
Conclusion: Identifying myxoma as a
rare pathology provoking CS in octageriansrequires a high index of
suspicion and diagnosis can be sometimes elusive. Differential diagnosis
of new onset cardiac dysrhythmias or even CS should include this
infrequent noxiousness. Clinical icon varies from asymptomatic to
causing severe morbidity and sudden death, with symptoms suggestive of
different cardiac entities. Surgical removal remains simplex process
offering curative treatment, an excellent prognosis and low recurrences
rates.
Cardiogenic shock was first defined as a rude
unhinging of the machinery of life. A more recent clinical definition
describes CS as decreased cardiac output and tissue hypoxia in the
presence of adequate intravascular volume. The most common cause of CS
is myocardial infarction with ST – segment elevation [1] whereas the
incidence in unstable angina is about 2.9% and 2.1% in non-ST-elevation
myocardial infarction [2]. CS leading to sudden deathis known to occur
in patients with primary cardiac tumors (Table 1) and is estimated to
constitute 0.01 to 0.005% of all sudden deaths [3]. Cardiac myxoma has
been reported as early as 1953 by Madonia et. al. [4] as a rare cause of
sudden cardiac death [4], but the first description of a left atrial
myxoma is accredited to King in 1845 [5]. It arises from the endocardium
as a lipidic cell mass embedded in a vascular myxoid stroma. Most
myxomas are sporadic and the cause is largely unknown. Myxomas are the
most common primary neoplasms of the heart comprising about 30-50%.
Approximately 75% are located in
the left atrial cavity, 23% in the right atrial cavity and about 2%
in the ventricular cavity [6,7]. The clinical presentation which
primarily depend on the cardiac chamber where they occur, varies from
asymptomaticincidental masses to serious life-threatening cardiovascular
complications and thus present as Achilles’ heel for early diagnosis.
Left atrial myxoma more often presents with symptoms and signs of
obstruction of the lesser circulation.

A 79-year-old woman was admitted to our hospital because
of a syncopal episode followed by altered mental status. The
patient was at her usual state of health with a six-month history
of exertional dyspnea managed as bronchial asthma, when she
suddenly lost consciousness and vomited while walking. She
was transferred by ambulance to the emergency department
within 40 minutes after the onset of symptoms. On arrival, she
said she did not have chest pain or headache, but she was unable
to provide other history. On examination, she was lethargic, with
periods of unresponsiveness. There was no sign of head trauma;
the remainder of the examination was normal. A radiograph of the
chest showed pulmonary vascular prominence with no evidence
of edema, infiltrate or effusion. An electrocardiogram revealed
sinus tachycardia with ST – segment elevation in V3 through
V6. Tests for creatinine kinase and troponin T were negative.
Transthoracic echocardiography showed hypokinesis of the
anterior, septal, and apical walls of the left ventricle. Overall left
ventricle systolic function was at the lower limit of the normal
range. The left atrium was dilated. There was a large pedunculated
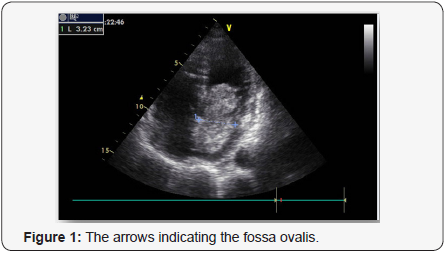
mobile echodensity in the left atrium that was attached to the
atrial septum (Figures 1 & 2).The mass was obstructing flow
with a mean trans mitral gradient of 17mm Hg, with a reduced
stroke volume and moderate to severe pulmonary hypertension
with an estimated right ventricular systolic pressure of 90 mm
Hg. Subsequent transesophageal views revealed a mass 7cm x
3cm x 3.4cm with its base attached to the interatrial septum.
The mass was highly mobile with multiple frondlike elements.
Color Doppler imaging showed no evidence of a patent foramen
ovale. Magnetic resonance imaging (MRI) of the brain revealed
no new infarcts. An emergency Interventional angiography
performed prior surgical excision and approximately 12 hours
after the onset of symptoms revealed an occlusive filling defect
in the proximal portion of the circumflexus coronary artery.
Balloon dilatation and DES implantation improved filling. The
patient was intubated, a nasogastric tube was placed and moved
to the operating room. Subsequently, surgical resection of the
left atrial mass was performed via transatrial surgical approach and the atrial septum defect repaired with a homologue patch.
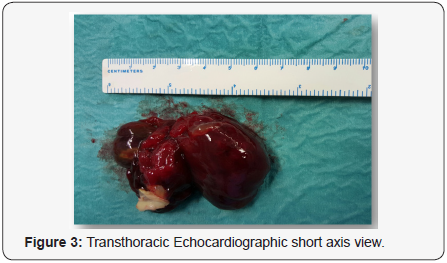
The resected mass was 7cm x 3cm x 3.4cm, with an irregular
frondlike surface (Figures 3 & 4). On microscopical examination,
the mass also had the morphologic features of a cardiac myxoma.


Immunohistochemistry showed that the cells were strongly
positive for vimentin, CD34 and for calretinin (29 kDa calbindin),
a calmodulin-like calcium -binding protein of unknown function.
Normal endocardium and organizing thrombi do not strain for
calretinin, whereas 80 to 100% of cardiac myxomas show strong
straining for this marker [8]. These histologic features are
diagnostic of cardiac myxoma [8,9].
Postoperatively she was initially treated with heparin
monitored three times per day, using the partial thromboplastin
time (aPTT) and the dose was adjusted to attain the target 50 –
60sec. On 3rd postoperative day she was set on apixaban (Eliquis
®) 2.5mg twice daily. The patient remained in the ICU for 1 day
and discharged to home on 7th postoperative day. The whole
postoperative period was uneventful.
The great majority of primary cardiac tumors are
histologically benign. Although benign tumors of the heart
are rare - autopsy studies suggest that the overall prevalence
is between 0.001 and 0.25% of the population [10,11] - it is
estimated that they account for more than 75% of primary
tumors of the heart. Although these tumors do not metastasize,
they can have catastrophic effects due to impairment of
cardiac structure and function, including the precipitation of
arrhythmias or embolism. Cardiac myxoma is the most common
benign primary tumor of the heart, accounting for up to 50% of
primary cardiac tumors.
The majority of myxomas are solitary and occur in the
left
atrium, typically in the zone of the fossa ovalis [12], as in this
case. It can be seen in patients between 3-83 years old, with
the majority presenting in fifth decade of life and just few cases
are mentioned presenting left atrial myxoma after the seventh
decade. Is most commonly seen in women with 90% being
solitary and pedunculated and 10% being familial, with an
autosomal dominant pattern of inheritance [13] When multiple,
recurrent, ventricular or familial myxomas are discovered,
there is cause to consider the diagnosis of Carney’s syndrome,
a familial syndrome characterized by multiple neoplasms [14].
The clinical features of atrial myxoma vary, as well as the size, with
most cases in literature referring to myxomas sized between
0.4 to 6,5 cm[15]. Large tumors are related to atrial fibrillation.
Diagnosis is often incidental and by symptomatic myxomas
challenging, as they often cause signs and symptoms that suggest
other, more common, conditions. This is understandable,
given the diverse array of signs and symptoms these patients
experience. Myxomas symptoms include dizziness, syncope and
dyspnea and are usually caused by intermittent obstruction of
the mitral valve by a prolapsing mass. In up to 50% of cases,
cytokine production by the tumor may result in fever, weight loss,
arthralgias or myalgias or even Raynaud’s phenomenon [16,17].
Obstructive symptoms appear at the 70% of patients with left
atrial myxoma and constitutional infarction estimated between
10 – 45%.Sudden cardiac arrest may be related with large
myxomas and complete obstruction of the mitral or tricuspid
valve orifices or myocardial infarction resulting from coronary
artery embolism [7,18]. There are 19 cases published since 1950
regarding sudden cardiac death due to myxomas [19].
Embolization, systemic or pulmonary, is identified as the
second most common manifestation that occurs in 30 to 40%
of cases, whereas 35% on left sided myxomas [20] and may
result from infected or uninfected thrombi on the tumor or
from release of fragments of the tumor itself. Depending on the
localization of the tumor emboli from left atrial myxomas cause
a greater diversity of complications, ranging from cutaneous
microinfarctions to transient ischemic attacks and acute stroke
syndromes [21,22].
Since myxomas develop within the heart, with the majority
of originating in the left atrium it is not surprising that
cardiac complications predominate and among them acutely
decompensated heart failure is the most frequent presentation
[23,24].
Echocardiography was reported to be the definitive
diagnostic modality. The sensitivity of transthoracic
echocardiography is about 95% determine accurately the
location, the size, the attachment and the mobility of the mass.
Transesophageal echocardiography has nearly 100% sensitivity
for cardiac myxoma. The tumor tissue manifests as spherical
– pedunculated mass attached to the endocardial surface with
hypoechoic areas [25].
Many cases required more than one diagnostic study, the
most common of which was computed tomography scans
that identified the mass incidentally and were subsequently
confirmed with echocardiography. In more recent reports, MRI
is increasingly mentioned as a modality to better characterize
tumor location and involvement preoperatively. Differences in
signal intensity between myocardium, tumor/thrombus is very
helpful, especially with the use of contrast agent like Gadolinium-
DTPA [26-28]. A cine MRI sequence is a very sensitive technique
to distinguish between an thrombus and a tumor, intra-cardiac
or intravascular.
Once the diagnosis of cardiac myxomas made, timely surgical
resection is required to prevent complications and secure
immediate and long-term excellent outcomes [22,23,24-29].
Operative mortality ranges from 0 to 3 % and recurrence rates
are between 1% and 3% for sporadic myxomas [30-33].
Myxomas are notoriously difficult to diagnose. The
presentation is varied and may be vague and diagnosis is
often delayed. The patients with cardiac myxomas often do not
have any obvious clinical signs. Relative hypotension and mild
tachycardia are most of the time present. The current tests of
choice are echocardiography and cardiac MRI with gadolinium
injection, which is useful to distinguish tumor from thrombus
because tumors are typically enhanced with gadolinium, whereas
thrombi are not. Therefore, myxoma should be considered in
patients without traditional cardiac risk factors who present
with progressive or acute dyspnea and clinical evidence of heart
failure, especially if a new cardiac murmur is detected. Myxoma
should always be on the differential for pulmonary embolism,
pulmonary hypertension and embolic strokes. Treatment
consists of prompt surgical excision.
Written informed consent was obtained from our patients
for publication of this case report and any accompanying images.
A copy of the written consent is available for review by the
Editor-in-Chief of this journal.
SID participated in sequence alignment, designing the case
report and drafting the manuscript. AT participated in the
design of the case report. KK participated in the design and
culled relevant information. DM participated in the design of the
case report, MS participated in the design of the case report, AR
participated in the design of the case report, KG participated in
the design of the case report, SM coordinated the preparation
of the case report. All authors read and approved the final
manuscript.
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php


Comments
Post a Comment