Dehydrated Human Amnion/Chorion Membrane Allograft Promotes Cardiac Repair Following Myocardial Infarctions-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
There is a rising need for novel therapies for
the treatment of acute myocardial infarction (MI). Dehydrated human
amnion/chorion membrane (dHACM) contains a unique array of regenerative
cytokines and growth factors, involved in the regulation of tissue
healing and modulation of inflammation; therefore, the ability of dHACM
to prevent cardiac damage or promote healing in an in vivo mouse model
of acute myocardial infarction was investigated. Acute left ventricular
MI was induced in mice by coronary artery ligation. dHACM patches were
then sutured to the MI zones, or the infarcted hearts were injected with
saline as a control.
After eight weeks post-treatment, dHACM-treated
and saline-treated hearts were histological sectioned and stained with
Masson’s trichrome to quantify infarct size or with antibodies for c-Kit
for recruitment of c-Kit positive stem/progenitor cells, Ki67 for
proliferation, TUNEL staining for apoptosis, and CD31 for vascular
endothelial cells. Results indicated that dHACM grafts promoted cardiac
repair in vivo after MI by reducing the size of fibrotic scarring. An
examination of cellular activity demonstrated an increased number of
c-Kit positive cells, greater cell proliferation with inhibition of
apoptosis, and an increased number of CD31 positive vessels in
dHACM-treated cardiac tissue. These results show that treatment with
dHACM in this animal model improved cardiac repair following MI through
multiple paracrine effects, including through improved blood supply and
recruitment of autologous stem cells.
Abbreviations: MI: Myocardial Infarction; PPCI: Primary Percutaneous Coronary Intervention; LV: Left Ventricular; LVAD: Left Ventricular Assist Device; dHACM: dehydrated Human Amnion/Chorion Membrane; FDA: Food and Drug Administration; AATB: American Association of Tissue Banks; HIV: Human iImmunodeficiency Virus; HTLV: Human T-Lymphotropic Virus; IACUC: Institutional Animal Care and Use Committee; NOD: Non-Obese Diabetic; SCID: Severe Combined Immuno-Deficiency; LAD: Left Anterior Descending; TUNEL: Terminal deoxynucleotidyl transferased UTP Nick End Labeling; ANOVA: Analysis of Variance; SCF: Stem Cell Factor
Introduction
Acute myocardial infarction (MI), more commonly known
as heart attack is a major consequence of cardiovascular disease and a
leading cause of morbidity and mortality worldwide. Acute MI occurs when
coronary occlusion causes diminished blood supply to the cardiac
tissue, and myocardial ischemia results in irreversible damage or cell
death [1]. Reperfusion therapy in the form of primary Percutaneous
coronary intervention (PPCI) has become the gold standard of treatment
for MI and has significantly improved short-term outcomes; however,
damage to the cardiac tissue still results in post-MI left ventricular
(LV) remodeling. LV remodeling occurs in response to loss of myocardial
function in the form of tissue scarring, dilatation, and hypertrophy,
and while remodeling is initially beneficial, this compensatory
mechanism can be maladaptive, ultimately leading to heart failure [2].
There are currently about 5.1 million Americans living with heart
failure with 825,000 new cases each year. It is estimated that the total
direct and indirect costs for heart failure in the United States exceed
$30 billion each year [3].
Therefore, there is a rising and urgent need for
novel therapies for the treatment of MI, particularly to treat or
prevent post-MI LV remodeling. Currently, pharmacologic, LV assist
devices (LVADs), and total heart transplantation are the only
forms of treatment, and none of them are without significant
limitations. For example, the efficacy of pharmacologic for the
prevention and treatment of post-MI LV remodeling and heart
failure is limited, LVADs are mainly used as a temporary bridge
to cardiac transplantation, and cardiac transplantation faces
limited donor availability and is only used as a last course of
action. Due to the inability of cardiac muscle to regenerate
following injury, stem cell therapy has been widely explored as
a treatment for cardiac disease, including numerous ongoing
clinical trials; however, significant gains in cardiac function have
yet to be established [4-6]. Poor engraftment and low survival
rates of injected cells represent major limiting factors in the
efficacy of cellular therapies for cardiac repair [4-7].
Dehydrated human amnion/chorion membrane (dHACM)
patches (EpiFix® and AmnioFix®, MiMedx Group, Marietta,
GA) are human allografts comprised of laminated amnion and
chorion amniotic membranes derived from the placenta. By
using a gentle cleansing and dehydration process (PURION®
Process), dHACM retains and preserves the native extracellular
matrix architecture and biological activity of the amniotic
membrane tissue [8-10]. The properties of PURION Processed
dHACM suggest that it may be a promising tissue for use in
treatment of acute MI. dHACM contains structural collagen and
additional extracellular matrix proteins, as well as a unique array
of regenerative cytokines and growth factors, involved in the
regulation of wound healing and inflammation [11-14]. These
soluble cues remain biologically active in dHACM and have been
shown to promote cell proliferation and endogenous growth
factor production, recruit stem and progenitor cells, and promote
angiogenesis [11-17]. Amniotic membranes are also known to
be immunologically privileged tissues with the ability to reduce
pain, prevent scarring, and control inflammation [18,19]. Recent
clinical trials have established the ability of dHACM to promote
healing in a variety of refractory wounds without recurrence in
long-term follow-up [20-22], while preliminary results suggest
that dHACM may also play a protective role in preventing the
progression of osteoarthritis [23]. These properties suggest that
dHACM may also possess the ability to either prevent damage or
assist cardiac repair following MI.
As a proof-of-concept evaluation, the ability of dHACM to
attenuate damage or promote cardiac repairin a mouse model of
acute myocardial infarction was investigated, including effects on
infarct size and cardiac remodeling. dHACM was examined for its
ability to reduce scar formation and inflammation, promote cell
proliferation and survival, attract endogenous stem/progenitor
cells, and enhance angiogenesis in vivo.
dHACM is a dehydrated human allograft comprised of
laminated amnion and chorion membranes derived from the placenta [8-10]. Human placentas were donated under informed
consent, following Caesarean sections, in compliance with the
Food and Drug Administration’s (FDA) Good Tissue Practice
and American Association of Tissue Banks (AATB) standards.
All donors were tested and confirmed to be free of infectious
diseases, including human immunodeficiency virus (HIV),
human T-lymph tropic virus (HTLV), hepatitis B and C, and
syphilis. Amnion and chorion were isolated from placenta and
processed with a proprietary PURION® Process that involves
gentle cleansing of the layers. The amnion and chorion were
then laminated to form the graft, and the graft was dehydrated
under controlled drying conditions [10]. Specific versions of
dHACM (EpiFix® and AmnioFix®, MiMedx Group) were used as
the test material in these studies; therefore, the results of these
studies apply only to PURION® Processed dehydrated human
amnion/chorion composite grafts (dHACM).
A previously established mouse myocardial infarction model
was used to examine the efficacy of dHACM in inducing cardiac
repair [24-27]. All marine experiments were conducted in
accordance with protocols approved by the University of Miami’s
Institutional Animal Care and Use Committee (IACUC). Either
8-week old female non-obese diabetic (NOD)/severe combined
immunodeficiency (SCID) mice or wild type mice, obtained from
the Jackson Laboratory (Bar Harbor, ME),were used to examine
the effects of human amniotic membrane implants in a mouse
xenograft model. Mice were anesthetized, orally intubated, and
underwent left thoracotomy in the fourth intercostals space. The
left anterior descending branch of the coronary artery (LAD)
was legated using an 8-0 nylon suture to induce MI.
Following acute myocardial infarction, mice were either
treated with a dHACM patch or with saline control treatment.
5 mm x 5 mm dHACM patches (EpiFix® or AmnioFix®) were
sutured to the MI zones. Mice receiving saline injections alone
were used as controls. The pericardium was re-draped over the
heart, and the chest was closed. Animals were placed into clean
cages and housed for the duration of 8weeks after recovery.
n=10 mice were used for treatment and control groups, and the
investigators were blinded to the type of treatment. Mice that
did not recover from the coronary artery ligation due to surgical
mortality were excluded from analysis. Animal deaths did not
appear to be related to dHACM treatment.
Eight weeks post-treatment, mice were euthanized, and
analyzed for cardiac remodeling. Hearts from each group were
fixed in 10% formalin, and embedded in paraffin. Paraffin
sections were subjected to Masson’s Trichrome staining, and
quantization of infarct size was performed. The average infarct
size was obtained by calculation of the average length of the
circumference in the infarct portion and the normal area. These indices of cardiac remodeling were compared among dHACM
treatment groups and saline controls.
To study if dHACM contributes paracrine factors that
resulted in enhanced cardiomyocyte proliferation, decreased
apoptosis, and induce recruitment of local c-Kit positive cardiac
progenitor cells or endogenous stem cells into the infarcted area,
tissue sections were stained with antibodies specific for Ki67
(proliferation marker), terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) for apoptosis, and c-Kit,
respectively.
To examine vascularization in cardiac tissue, histological
tissue sections were also stained with antibodies for CD31
to identify vascular endothelial cells. The sections were
counterstained for troponin (red) for myocardial tissue and
with DAPI (blue) for cell nuclei. The total number of CD31-
positivevessels per 1.0x104 μm2 of MI area was counted and
compared in samples across different groups, as a measure of
vascularity.
Values were reported as mean ± standard deviation.
Statistical analyses were performed using analysis of variance
(ANOVA) with Tukey’s post hoc test for pair wise comparisons.
Significant differences were assigned when p≤0.05.
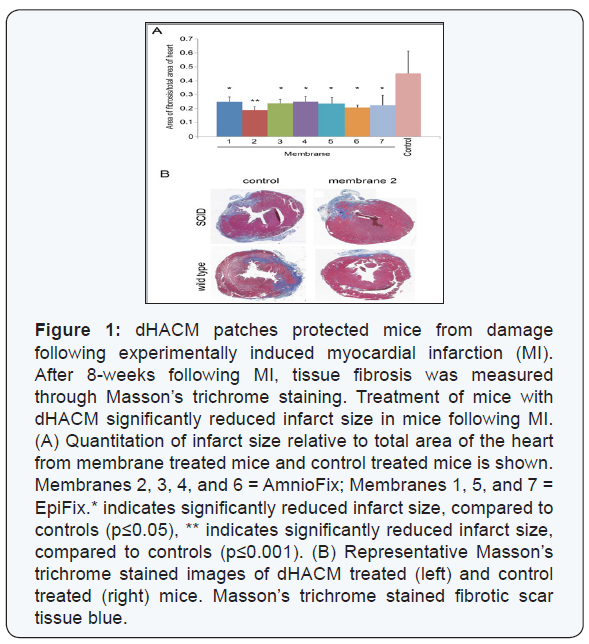
A collagenous scar was visible in the infarct sites, indicating
that coronary artery ligation resulted in cardiac fibrosis (Figure1), blue. dHACM treated mice showed a significant decrease of
approximately 50% in infarct area following MI, compared to
control (saline) treated mice p≤0.05; (Figure 1). There was no
statistically significant difference in infarct size between the
EpiFix and AmnioFix-treated mice; therefore, all dHACM patch
results were combined for the following analyses. Additionally,
reduction in infarct size was seen in both dHACM-treated NOD/
SCID and wild type mice, indicating that the dHACM tissue was
well tolerated by wild type mice.
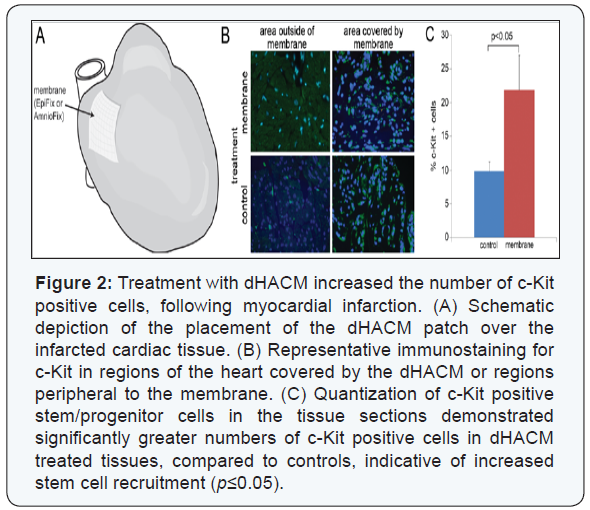
A significant increase in the stem cell marker c-Kit was
observed in dHACM-treated hearts, compared to the control
group p≤0.05; (Figure 2). Significantly greater numbers of c-Kit
positive cells were present both in the region directly in contact
with the dHACM, as well as regions of the heart peripheral to the
membranes. c-Kit is a cell surface receptor for stem cell factor
(SCF), and it is a marker for hematopoietic stem cells and cardiac
stem/progenitor cells [28,29]. Therefore, the increased presence
of c-Kit positive cells indicated that dHACM treatment promoted
the recruitment of endogenous c-Kit-positive stem cells either
from the bone marrow or locally from the heart to the site of
infarction following acute MI.
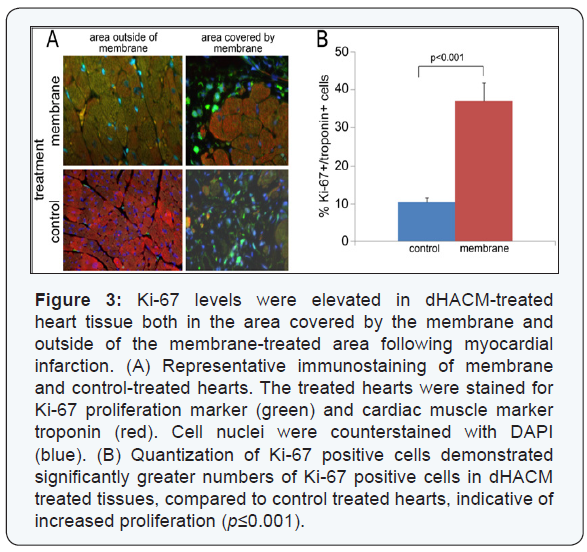
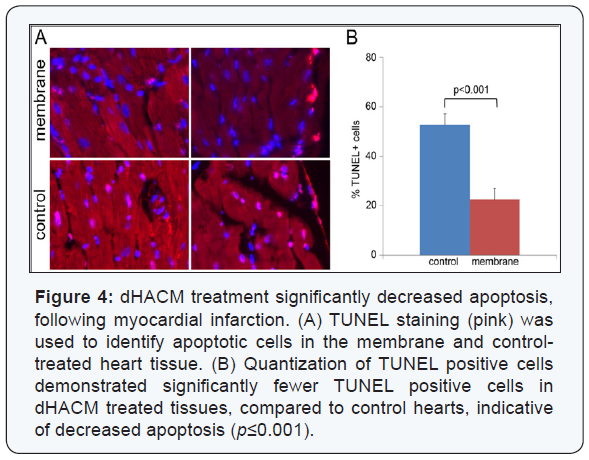
There was a significant increase in the cell proliferation
marker Ki-67 in the membrane-treated hearts, compared
to the control group p≤0.001; (Figure 3). Ki-67 expression
also extended beyond the region in direct contact with the
membranes into the distal heart tissue. Ki-67 is a nuclear
protein that is a marker for proliferating cells [30]. Additionally,
there was a significant decrease in apoptosis in the membranetreated
hearts, compared to the saline treatment (p≤0.001),
as measured by TUNEL staining (Figure 4). TUNEL staining is indicative of DNA fragmentation, correlated to cell apoptosis.
Increased Ki-67/troponin double-positive cells and decreased
TUNEL/troponin double positive cells indicated that dHACM
treatment promoted cardiomyocyte proliferation and inhibited
cardiomyocyte apoptosis, concurrently. These data suggest
that paracrine signals from the dHACM patches promoted cell
proliferation, inhibited apoptosis, and enhanced cell survival
following MI.
An increased number of blood vessels were found in the
membrane-treated hearts, compared to the control mice
(p≤0.05), as indicated by staining for CD31 positive vessels
(Figure 5). CD31 is a cell surface marker for vascular endothelial
cells. An increase in CD31 positive vessels indicated enhanced
vascularization in the cardiac tissue, suggesting that dHACM
treatment increased angiogenesis and neovascularization within
the cardiac tissue following MI.

Using an established coronary artery ligation model of acute
left ventricular myocardial infarction, these results represent
proof-of-concept that treatment with dehydrated human
amnion/chorion membrane (dHACM) grafts maybe capable
of promoting cardiac repair in vivo after MI. dHACM treatment
reduced the size of fibrotic scarring after MI, indicating that
dHACM had a protective effect on the ischemic tissue. Fibrosis
of cardiac tissue is an important aspect of cardiac remodeling,
as the collagenous scar remains stiff and unable to contract.
Cardiac scar tissue leads to further remodeling, including left
ventricular hypertrophy, a compensatory mechanism which can
ultimately result in heart failure.
As an initial investigation to determine the mechanism of
action for dHACM-mediated cardiac repair, an examination
of cellular composition and activity in the cardiac tissue was
performed to examine a number of events which may promote
cardiac repair. An increased number of c-Kit positive cells
were identified in dHACM-treated cardiac tissue, suggesting
the dHACM may recruit c-Kit positive hematopoietic stem cells
and/or cardiac stem cells to the site of injury, stimulating repair.
Additionally, dHACM promoted cell proliferation while inhibiting
apoptosis, as demonstrated by Ki-67 and TUNEL staining,
respectively, demonstrating enhanced cell survival. Finally
dHACM treatment resulted in an increased number of CD31
positive vessels in cardiac tissue after MI, indicative of enhanced
angiogenesis. Together these results suggest that treatment
with dHACMmay improve cardiac repair following MI through
multiple paracrine effects. dHACM protected cardiac cells and
tissue from damage in vivo, possibly through improved blood
supply to the infarcted tissue. Additionally, dHACM recruited
autologous stem/progenitor cells into the ischemic cardiac
tissue. Recruitment of autologous cardiac stem cells possesses
tremendous potential to promote repair and regeneration of
cardiac tissue.
The properties of dHACM suggest that it may be a potential
therapeutic biomaterial for MI treatment as a cardiac patch.
dHACM contains a vast array of growth factors and cytokines
within a naturally-derived extracellular matrix scaffold.
The PURION Process preserves the bioactivity inherent
to amniotic membrane tissue, including a unique array of
regenerative cytokines and growth factors. To date, over 226
growth factors, cytokines, and regulatory molecules have been
identified in dHACM tissues [11-14,31]. These soluble cues
remain biologically active in dHACM, combining to promote
cell proliferation and endogenous growth factor production of
various cell types, recruit stem cells to the site of implantation,
and promote angiogenesis in vitro and in vivo [11-17]. dHACM is
also inherently biocompatible and non immunogenic. Amniotic
membrane is an immunologically privileged tissue that acts as
a natural barrier between the mother and developing fetus, and
treatment with amniotic tissue has demonstrated the ability
to reduce pain, prevent scarring, and modulate inflammation
[18,19]. Therefore, dHACM tissue represents a structural patch
that contains collagen and extracellular matrix which may also
serve as a biologically active bandage for the infarct zone.
This is the first report that dHACM has positive effects in
protecting tissue or promoting repair following acute myocardial
infarction. A previous study by Cargnoni et al. [32] demonstrated
that fresh human amniotic membrane (used within 24 hours)
significantly reduced post-ischemic cardiac injury using a similar
rat coronary artery ligation model [32]. In the Cargnoni study,
amniotic membrane-treated rats demonstrated preservation of
cardiac dimensions and improved cardiac contractile function,
in terms of higher left ventricle ejection fraction, fractional
shortening, and wall thickening, within 7 days after application
and persisting for at least 2 months [32]. Additionally, no
engraftment of amniotic cells was detected into host cardiac
tissues, suggesting that repair was likely mediated by the release
of cardio protective soluble factors to the injured ischemic tissue
[32]. Since dHACM used in this study is dehydrated and does
not contain viable cells but retains and preserves the bioactive
cellular components of the native amniotic membrane, it is
likely that the cardiac repair demonstrated here is also mediated
through similar soluble signals.
These results suggest that dHACM may warrant further
investigation for use in cardiac repair following myocardial
infarction. Follow up studies will include demonstrating
improvement of important functional outcomes such as left
ventricular dimensions, contractility, and ejection volume,
as well as demonstrating efficacy in a large animal model of
MI, such as a porcine model. Additional experiments are also
required to further elucidate the roles of stem cell recruitment
and neovascularization in cardiac repair, including potential
interactions and characterization of the molecular mechanisms
underlying the efficacy of dHACM in the repair of other tissues.
However, this work is a first step suggesting that dHACM
allografts possess tremendous potential in promoting cardiac
repair following acute myocardial infarction.
dHACM allografts appeared to promote cardiac repair after
MI by reducing the size of fibrotic scarring in a mouse model.
Application of dHACM patches stimulated an increase in c-Kit
positive stem cells, cell survival, and vascularization in infarcted
cardiac tissue. These results suggest that treatment with dHACM
following MI may improve cardiac repair through multiple
paracrine effects, including through improved blood supply and
recruitment of autologous stem cells.
dHACM allografts appeared to promote cardiac repair after
MI by reducing the size of fibrotic scarring in a mouse model.
Application of dHACM patches stimulated an increase in c-Kit
positive stem cells, cell survival, and vascularization in infarcted
cardiac tissue. These results suggest that treatment with dHACM
following MI may improve cardiac repair through multiple
paracrine effects, including through improved blood supply and
recruitment of autologous stem cells.
dHACM allografts appeared to promote cardiac repair after
MI by reducing the size of fibrotic scarring in a mouse model.
Application of dHACM patches stimulated an increase in c-Kit
positive stem cells, cell survival, and vascularization in infarcted
cardiac tissue. These results suggest that treatment with dHACM
following MI may improve cardiac repair through multiple
paracrine effects, including through improved blood supply and
recruitment of autologous stem cells.
For more articles in Open Access Journal of
Cardiology & Cardiovascular Therapy please click on: https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment