Transthoracic Echocardiographic Guidance for Percutaneous Patent Ductus Arteriosus Closure in Pediatric Patients-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY &
CARDIOVASCULAR THERAPY
Abstract
Background:: Patent ductus
arteriosus (PDA) is a common congenital cardiac disorder; the treatment
of choice is fluoroscopy-guided transcatheter occlusion. Closure of PDA
under transesophageal echocardiography (TEE) with Trans aortic imaging
guidance was studied in adult patients. In pediatric patients,
transthoracic echocardiography (TTE) provides excellent images for PDA
and may replace fluoroscopy as an imaging tool to guide PDA closure.
Purpose: The goal was to
study the feasibility of device closure under TTE guidance in patients
who are not candidates for transfer to the cardiac catheterization
laboratory or in those with contraindications for contrast and/or
radiation applications.
Methods: Eighteen patients (7
males, median age 7 months, median weight 8 kg) underwent TTE guidance
device closure of PDA from July 2013 to May 2015. Conscious sedation was
used in 17 patients-one patient was sick and ventilated in the
intensive care unit. The antegrade approach with partial fluoroscopy was
used in eight patients, and the retrograde approach without fluoroscopy
in 10 patients.
Results: The median procedure
time was 30 minutes (13-45 min). The median fluoroscopy time was 2.2
minutes (0-13 min). The PDA size was small in 13 cases and moderate in
five subjects. Immediate successful closure was achieved in 17 patients.
One patient had device emobilization after the release of the device
during the retrograde approach. The device was successfully retrieved
under fluoroscopy.
Conclusion: Transcatheter PDA
device closure under TTE guidance is feasible and recommended in
selected patients and can replace fluoroscopy.
Abbreviations: PDA: Patent Ductus Arteriosus; TEE: Transesophageal Echocardiography; TTE: Transthoracic Echocardiography; AVP2: Amplatzer Vascular Plug type 2; MPA: Main Pulmonary Artery; ADOS: Amplatzer Duct Occluder additional Size; ADO II: Amplatzer Duct Occluder type II
Introduction
Transcatheter closure of patent ductus arteriosus
(PDA) under fluoroscopy guidance is the method of choice in pediatric
subjects over the last four decades (Figure 1). However, there is an
urgent need for alternative imaging methods in patients with
contraindications to fluoroscopy.
Transthoracic echocardiography (TTE) is already used
for guidance of secudum atrial septal defect closure [1,2]. In addition,
this method was successfully used to guide many other procedures. This
makes it an attractive tool, especially in
pediatric patients, where TTE provides excellent images for PDA (Figure
1).
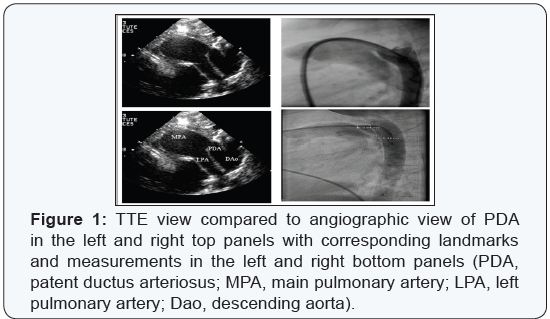
In adult patients with PDA, transesophageal echocardiography
(TEE) with transaortic imaging is an important alternative
and offers good image quality [3]. However, it is disfavored in
pediatric patients because of the need for general anesthesia.
Nevertheless, a few reports described TTE as a tool to monitor
PDA closure before, during, and after the procedure [4-9]. We
describe our experience in using TTE to guide the transcatheter
closure of PDA with and without fluoroscopy.
A total of 18 patients (7 males) underwent TTE guidance
device closure of PDA between July 2013 and May 2015 at Prince
Sultan Cardiac Center. The median age of patients was 7 months
(range 4-23 months) and the median weight was 8 kg (range
3.2–11 kg). All patients received a detailed echocardiographic
evaluation of the duct morphology as part of the institutional
protocol using broadband 3-8 MHZ (AEPEC, Philips, USA/
Holland) and 5-12 MHZ frequency transducers. The duct
diameter was measured in a high parasternal long-axial view at
the insertion of PDA to the pulmonary artery. The measurements
were based on zoomed two-dimensional echocardiographic
images and not on the color Doppler jets width. Because this
measurement varies with different phases of the cardiac cycle,
the maximum measurement diameter at the level of pulmonary
artery insertion was reported as the narrowest diameter (duct
diameter) and the widest diameter at the aortic side was
reported as ampulla and the length of the duct is the distance
between ampulla and PDA-MPA junction (Figures 2 ).
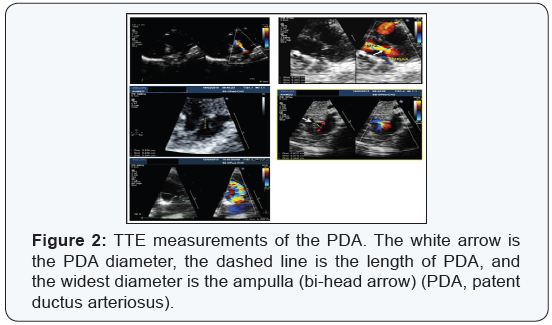
The ductal ampulla or the diverticulum was defined in the
high parasternal long-axis view or in the supra-sternal longaxis
view. The ductal ampulla was considered adequate if its
maximal dimension in the long axis view was greater than twice
the measured ductal diameter. Careful 2-D and Doppler imaging
of the pulmonary artery branch origins was also routinely
performed. Multiple and repeated measurements were collected
and the most concordant value was reported.
In the cardiac catheter laboratory, the femoral vein (in seven
patients) and the jugular vein (in one patient) were accessed with a 5F sheath. The end and side hole catheter was used to
check the hemodynamics and to approach the PDA under
fluoroscopy guidance, followed by crossing the PDA via a soft
0.035-inch exchange length Terumo wire (Terumo Corp., Tokyo,
Japan). The catheter was then replaced with a long sheath (size
5 or 6F). The devices were then deployed under TTE guidance
(Figure 3).
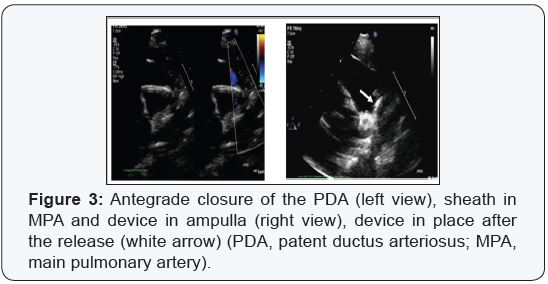
We used the following devices: Amplatzer duct occluder
type I (ADO I, ST. Jude Medical corp. , St. Paul, Minnesota, USA
), Occlutech (Occlutech, Jena, Germany), and Amplatzer vascular
plug type 2 (AVP2, ST. Jude Medical corp. , St.Paul, Minnesota.
The disc was released in the ampulla and then pulled until the
rest of the device seen in the main pulmonary artery (MPA)
under 2D and color doppler transthoracic echocardiography
guidance, followed by complete release of the device with no
need for contrast angiography or arterial access.
In the catheter laboratory, the femoral artery was accessed
in 10 patients who have small to moderate PDA using a 4F
short sheath to cross the PDA via a 4F end hole catheter. A 4F
long sheath then has replaced the catheter over a soft 0.035
exchange length Terumo wire (Terumo Corp., Tokyou, Japan).
followed by device implantation. We used the following devices:
Amplatzer duct occluder additional size (ADOS, ST. Jude Medical
corp. , St. Paul, Minnesota, USA), Amplatzer duct occluder type II
(ADO II, ST. Jude Medical corp. , St. Paul, Minnesota, USA ), and
AVP2 ( ST. Jude Medical corp. , St. Paul, Minnesota, USA ).

This procedure consists of the release of the first disc in the
main pulmonary artery, followed by pulling the sheath with the device toward the PDA and aorta. The disc is then fixed against
the PDA and the rest of the device is released in the ampulla.
This was visualized with color Doppler ultrasonography, and a
waggle movement was applied to ensure device stability. The
device position and shape was reassessed after the release-all
under TTE guidance (Figure 4). The contrast angiography or
fluoroscopy was not necessary. However, a femoral venous line
was secured with a 20G needle (Vygon, Aachen, Germany) for
the emergencies.
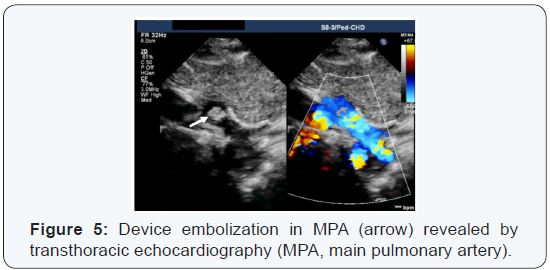
One patient was assessed before the procedure for a
retrograde approach with the PDA device; the pre- and intraprocedural
measurements were consistent with the use of a 4×4 ADOS. However, after the device release, it was embolized
immediately in the main pulmonary artery (Figure 5). Therefore,
fluoroscopy was used to define the exact location of the device.
A short sheath was inserted in the femoral vein, which was
then replaced with a long sheath for the device retrieval. The
procedure was very successful and uneventful. Finally, a 6×4 ADO
I was used for ante grade closure of the PDA under fluoroscopy
guidance (total fluoroscopy time was 13 min).
All procedures were performed under conscious sedation
except for one patient who was ventilated in the intensive care
unit before the procedure. Two patients had chronic renal
impairment and two patients showed the relapse of leukemia.
The median procedure time was 30 min (range 13-45 min) and
median fluoroscopy time was 2.2 min (range 0-3 min), except for
one patient, in whom the device embolized (this consumed 13
min because of the retrieval of the device and re-implantation of
a second one under fluoroscopy guidance). We found small PDAs
in 13 subjects and moderate PDAs in five. Immediate successful
closure was achieved in all subjects except one. Complete
closure was documented by color Doppler ultrasonography on
the second day before discharge (patients’ characteristics were
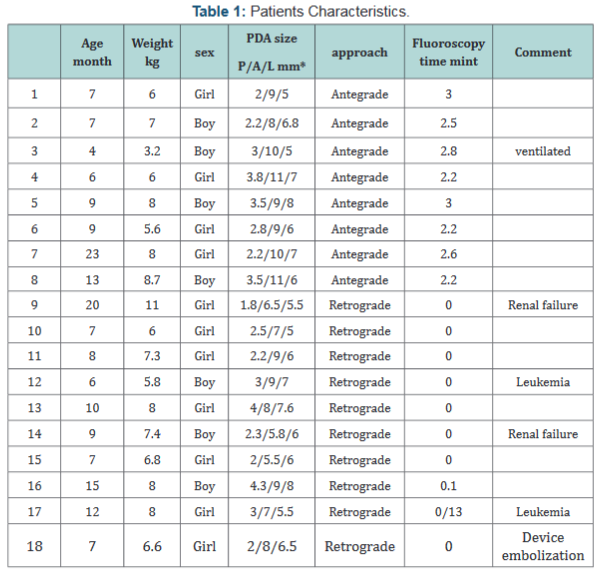
shown in the following Table 1).
Different devices were used according to the approach, size,
and shape of the PDA. However, the ADO I and ADO II, Occlutech
duct occluder device and AVP2 were mainly used with the
antegrade approach. The ADOS, ADO II, and AVP2 were used in the retrograde approach, because of the availability of two
discs in these devices, which allow the release of one disc in
the MPA and the other in the ampulla. No immediate or early
complications were found in 17 patients.
Transcatheter closure of PDA is routinely performed under
fluoroscopic guidance. However, alternative methods of
guidance are recognized and applied worldwide. The use of TTE
to guide atrial septal defect secundum closure suggests that it
can also be used in other congenital heart defects [1,2]. The 3-D
echocardiography was more accurate than 2-D echocardiography
in determining the length and the location of the PDA ampulla.
However, the use of 3-D echocardiography to assess the small
vascular structures (e.g., PDA) in children with rapid heart rates
are still of limited clinical value [10]. TEE can be an alternative
with good image quality, but it is primary applied in adult
population and animals. It is disfavored in pediatric populations
because of the need for general anesthesia [3]. TEE monitoring
played an important role in PDA ligation and assessed residual
blood flow. It also monitored the pulmonary artery and the
aortic valve [11].
Retrograde imaging with the 8F intracardiac
echocardiographic catheter (Acu Nav, Acuson-Siemens, Mountain
View, CA, USA) from the femoral artery into the descending aorta
can be used for anatomical characterization and sizing of the PDA
in adults [12]. Transaortic phased-array imaging can effectively
assist the percutaneous PDA device closure. This may reduce
procedure time, limit radiation exposure, and lower the amount
of contrast agent administered [13]. In pediatric populations,
transcatheter PDA closure under fluoroscopy guidance has
been preferred for the last 40 years [14]. However, a study
reported in 2007 the use of TTE for transcatheter PDA closurein
neonates [4]. The success of this method [5] suggested that it
could replace fluoroscopy and guide this procedure at least in
some situations when the patients are too sick for the cardiac
catheter laboratory, are at high risk, or have contraindications
for contrast angiography or / and radiation [6-8].
Baykan et al. [9] described percutaneous PDA closure via
ADO I using the femoral venous access without the need for
arterial access or contrast angiography. They deployed the
devices under TTE guidance, and this technique can be used in
patients whose femoral artery could not be accessed or if the
access is impossible / contraindicated [9]. In our study, we used
the same classification of PDA size as reported by Rao [14]. We
used both femoral veins and arteries to access the PDA with
different approaches as described above. We found that TTE
images were very helpful in guiding the device deployment. With
more experience, we found that even the course of the catheter
and the sheath could be visualized via TTE. The challenges in
visualizing the catheter course inside the cardiac structure by
TTE motivated our use of arterial access to cross the PDA via a
straight course from the aorta.
The TTE-based PDA measurements (diameter, length, and
ampulla) were feasible. Previous fluoroscopy procedures showed
that TTE is largely comparable to angiography measurements with a difference of 0.5-0.8 mm. We used the average of multiple
measurements from different images in the presence of at least
two image readers during the procedure. We used devices that
were 2 mm larger than the maximum diameter of the narrowest
end of PDA, at the opening into the MPA.
It is difficult to explain the reasons for device embolization.
However retrospective review of the patient’s images suggests
some theories behind the embolization:
- The second disc of the device might be partially released in the MPA. Because it is short arterial duct it was difficult to realize the protrusion of the second disc.
- The PDA size was larger than what we measured.
- There is possibility that the PDA is a stretchable type and the size might be changed with the catheter and sheath manipulation.
- It might be that both discs were released in the MPA due to the presence of short PDA. Howevers, we managed to retrieve the device and close the PDA with a larger device under fluoroscopy guidance, which made the procedure longer.
This is the limitation for the success of the procedure in the
current time.
Transcatheter PDA closure guided by TTE is a feasible and
very promising method as an alternative to fluoroscopy. In
the future, certain patients may be helped with this approach.
More clinical experience will further improve the technique
and minimize the embolization rate. While visualization of the
embolized device is feasible, the retrieval of the device needs
fluoroscopy at least in the present time.
I would like to thank all the pediatric catheter laboratory
team in PSCC for their assistance and contribution in the team
work to provide the best care to the patients.
All study participants provided informed consent, and the
study design was approved by the appropriate ethics review
board.
For more articles in Open Access Journal of Cardiology & Cardiovascular
Therapy please
click on:
https://juniperpublishers.com/jocct/index.php
https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment