Does the Use of Local Anesthesia with Conscious Sedation Rather Than General Anesthesia Improve the Outcomes of Transfemoral Transcatheter Aortic Valve Replacement?-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Mini Abstract
According to the results of this study, TF-TAVR under
LACS seems to be as safe and effective as it is under GA.LACS could
reduce infectious complications and median hospital stay after TF-TAVR.
Background: Transcatheter aortic
valve replacement is a proven treatment for patients with aortic stenos
is who are considered at high-risk or with contraindications for
surgical aortic valve replacement. General anesthesia (GA) and local
anesthesia with conscious sedation (LACS) have been described for this
procedure. However, there is no randomized study that looked for the
benefit of using LACS for this procedure. The anesthetic management
during TAVR is still controversial, and some centers are still
performing it mainly under GA. This study aimed to look for benefits of
LACS over GA regarding the outcomes of transfemoral transcatheter aortic
valve replacement (TF-TAVR).
Methods: We analyzed data of our
patients’ cohort, who underwent TF-TAVR between February 2010 and
October 2011 at La Pitie-Salpetriere Hospital, those patients were part
of the French Aortic National Core Valve and Edwards 2(FRANCE2). The
procedure was performed either under GA or LACS. Two TAVR systems were
used. Device success, safety and efficacy endpoints, prosthetic valve
performance, complications and hospital stay were compared between LACS
and GA groups.
Results: From 78 consecutive
patients who underwent TF-TAVR, 40 received LACS and 38 received GA.
Device success rate was 90.0% in the LACS group and 92.1% in the GA
group (p=1.00). Thirty-day mortality rate was 14.1% overall, with no
significant difference between groups (p=0.29). There were no
significant differences in safety and efficacy endpoints, the prosthetic
valve performance, therapy-specific and prosthetic valve associated
complications between the two groups. Infectious complications rate was
significantly lower (p=0.04) and the median hospital stay was
significantly shorter (p=0.04) in LACS group.
Conclusion: In our experience,
TF-TAVR performed under LACS seems to be as safe and effective as it is
under GA. LACS could reduce infectious complications and median hospital
stay after the procedure.
Abbreviations: AR: Aortic Regurgitation; AVA: Aortic Valve Area; AVB: Atrioventricular Block; AVR: Aortic Valve Replacement; CABG: Coronary Artery Bypass Graft; COPD: Chronic Obstructive Pulmonary Disease; Euro SCORE: European System for Cardiac Operative Risk Evaluation; GA: General Anesthesia; HF: Heart Failure; LACS: Local Anesthesia with Conscious Sedation; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; MI: Myocardial Infraction; MR: Mitral Regurgitation; SAVR: Surgical Aortic Valve Replacement; TAVR: Transcatheter Aortic Valve Replacement; TF-TAVR: Transfemoral Transcatheter Aortic Valve Replacement; VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged
as a credible alternative therapy for patients with aortic stenosis
who are considered at high-risk or with contraindications
for surgical aortic valve replacement (SAVR) [1]. Centers still
disagree about the preferred anesthetic technique for TF-TAVR
[2]. Most teams perform the procedure under general anesthesia
(GA), although local anesthesia with conscious sedation (LACS)
may suffice for the transfemoral approach [1].
The subanalysis of the French Aortic National Core Valve and
Edwards 2 Registry (FRANCE2) [3] compared clinical outcomes
and safety of TAVR Under GA versus local anesthesia, and didn’t
demonstrated a clinical benefit of the local anesthesia over the
GA. However, the lack of finding a benefit of performing the
procedure under local anesthesia could be due to the nationwide
nature of the registry and to the fact that many outcomes were
self-reported and not independently adjudicated. The purpose
of this study was to report the analysis of the clinical outcomes
of transfemoral transcatheter aortic valve replacement (TFTAVR)
under LACS compared to GA performed in our Centre,
the patients included here were also part of FRANCE 2 registry
[3]. The collect of clinical endpoints was more extensive in the
present study.
We analyzed data of our cohort of patients who underwent
TF-TAVR with either the Medtronic Core valve System or the
Edwards Sapien valve, who were part of the FRANCE 2 registry
[4], from February 2010, to October 2011. From 102 consecutive
patients with severe Aorticstenosis who underwent TAVR at La
Pitie-Salpetriere University Hospital, 78 patients had TF-TAVR. All
consecutive patients referred to our center for the management
of symptomatic severe native aortic stenosis with high risk for
open-heart surgery as expressed by a logistic European System
for Cardiac Operative Risk Evaluation (Euro SCORE)≥20% or with
specific surgical contraindications as described previously [1,5]
and who had a TF-TAVR were included. All these patients met the
eligibility criteria defined for TAVR [1]. The decision to perform
the procedure was made by a multidisciplinary team involving
an interventional cardiologist, a cardiothoracic surgeon, a
cardiologist, cardiac anesthesiologist, an echocardiographist and
an imaging specialist. Written informed consent was obtained
from each patient enrolled. The study protocol was approved by
the local ethics committee.
All patients had trans thoracic echocardiography or
transesophageal echocardiography (TEE) when required,
Doppler ultrasound of the supra-aortic arteries, coronary
angiography, and multi slice computed tomography of the aortic
root and aortoiliofemoral system. Pulmonary function test was performed if there was a history of respiratory disease. Assessing
the feasibility of TF-TAVR was mainly based on the results
of multi slice computed tomography. For all patients, aortic
annulus measurement was performed by the multi slices canner.
Patients were excluded if this diameter was <18or>25mm, or
if they had contraindications to the transfemoral approach [6].
A preanesthetic consultation was performed at least 48 hours
before the procedure.
All TF-TAVRs were performed in a hybrid operating room.
Detailed technical aspects of the TAVR procedure have been
previously described [1,7]. An interventional cardiologist and
a cardiac surgeon actively took part in the procedure. Aortic
annul us dimensions determined by the multi slice computer
tomography guided the choice of the device. Four types of
prosthesis were used (Edwards SAPIEN valve [ESV, Edwards Life
sciences Inc. CA, USA] and Medtronic Core valve Revalving system
[MCRSTM, Core Valve Inc. Irvine, CA, USA] were initially used,
gradually replaced by subsequent generations SAPIENXT and
Core valve Accutrack). The procedure was guided by fluoroscopy
and angiography. Immediately after TAVR, autography, and TEE
when ever available, were performed to assess the presence of
aortic regurgitation, the patency of the coronary arteries and to
rule out complications [1].
The procedure was performed either under GA or LACS. The
choice of the type of anesthesia used for every patient was left
to the discretion of the physician (s) involved. During the initial
phase of the study, our practice of performing TF-TAVR was to
operate mainly under GA then gradually we shifted to LACS.
Anesthesia personnel involved in the procedure were a cardiac
anesthetists and an experienced nurse anesthetist.
Detailed technical aspects of anesthetic and per operative
management of patient sunder going the TAVR procedure
have been previously reported [8,9]. GA was induced with an
intravenous bolus of etomidate (0.25-0.4mg/kg) or propofol
(1.2-2.5mg/kg) associated to either remifentanil (1μg/Kg),
alfentanil (50-100μg/Kg), sufentanil (0.5-2μg/Kg) or fentanyl
(2-50μg/Kg). The choice of anesthetic drug was at the discretion
of the anesthesiologist. It was facilitated by atracurium (0.3-
0.6mg/Kg). Anesthesia was maintained with either a continuous
infusion of propofol or in etomidate or an inhaled agent
(desflurane, sevoflurane, or isoflurane) and the opioids used
for induction. For LACS, ilioinguinal/Iliohypogastric blocks and
infiltration with a mixture of lidocaine and ropivacaine were
performed by the cardiac anesthesiologist approximately 30
minutes before the onset of the procedure. All patients under
went sedation with remifentanil (target-controlled infusion of
1-3ng/mL adjusted to obtain a Ramsay score of 2-3). If maximal
dose of remifentanil was insufficient, supplementation with a
target-controlled infusion of propofol was performed.
Clinical and par clinical data have been collected partially
prospectively taken from our center’s registry which is part
of FRANCE 2 registry, and completed retrospectively from the
health records for more extensive endpoints collect. We assessed
base line patient characteristics, procedural characteristics and
anesthesia-related information. Post-procedural outcomes,
defined according to the Valve Academic Research Consortium
criteria (VARC) [10], were collected over a follow-up of 30-days
and were compared between the two groups: LACS versus GA.
All statistical analyses were performed with the use of
IBMSPSS Statistics version 20 (SPSS, Chicago, IL, USA). Continuous
variables were presented as means ± standard deviations when
normally distributed and as medians (25th-75thpercentiles) when not normally distributed. They were tested for differences
with the Student’s t-test or non-parametric tests respectively.
Categorical variables, were expressed as percentages, and were
compared with the use of the Pearson’s chi-square or the Fisher’s
exact test, as appropriate. All statistical tests were 2 sided and a
p value<0.05 was considered significant.

The procedural characteristics and device success rate are
shown in (Table2). Overall, device success was achieved in
91.0% of patients, and was similar in the two groups (p=1.00).
Three cases had device failures in GA group, they were due to
a prosthetic aortic regurgitation grade ≥ 3 in 2 patients who
had asymmetric aortic annulus and to an incomplete opening
of the prosthesis secondary to calcareous fragments at the native valve in another patient. In LACS group, four cases didn’t
meet device success criteria, two of them had prosthetic aortic
regurgitation grade ≥ 3 due to prosthesis malposition, one case
had left ventricular wall perforation and unfortunately had
a peril-procedural death, and in one case the iliac artery was
impassable and required conversion to on-pump SAVR.
All P values are for between-group comparisons.
There were no significant differences in terms of efficacy
and safety endpoints between the two groups (Table 3). The
Valve Academic Research Consortium combined safety endpoint
(all-cause mortality, major stroke, peril-procedural myocardial
infarction, life-threatening bleeding, and kidney injury RIFLE
stage3) was comparable for the two groups (10.0%[n=4] in
LACS group vs.21.1% [n=8] in GA group, p=0.22). All-cause30-
daymortality rate (including intra-hospital mortality) was
14.1% (n=11) for the whole of the series, 10.0% (n=4) in LACS
group vs. 18.4% (n=7) in GA group, (p=0.29). Overall 10.3%
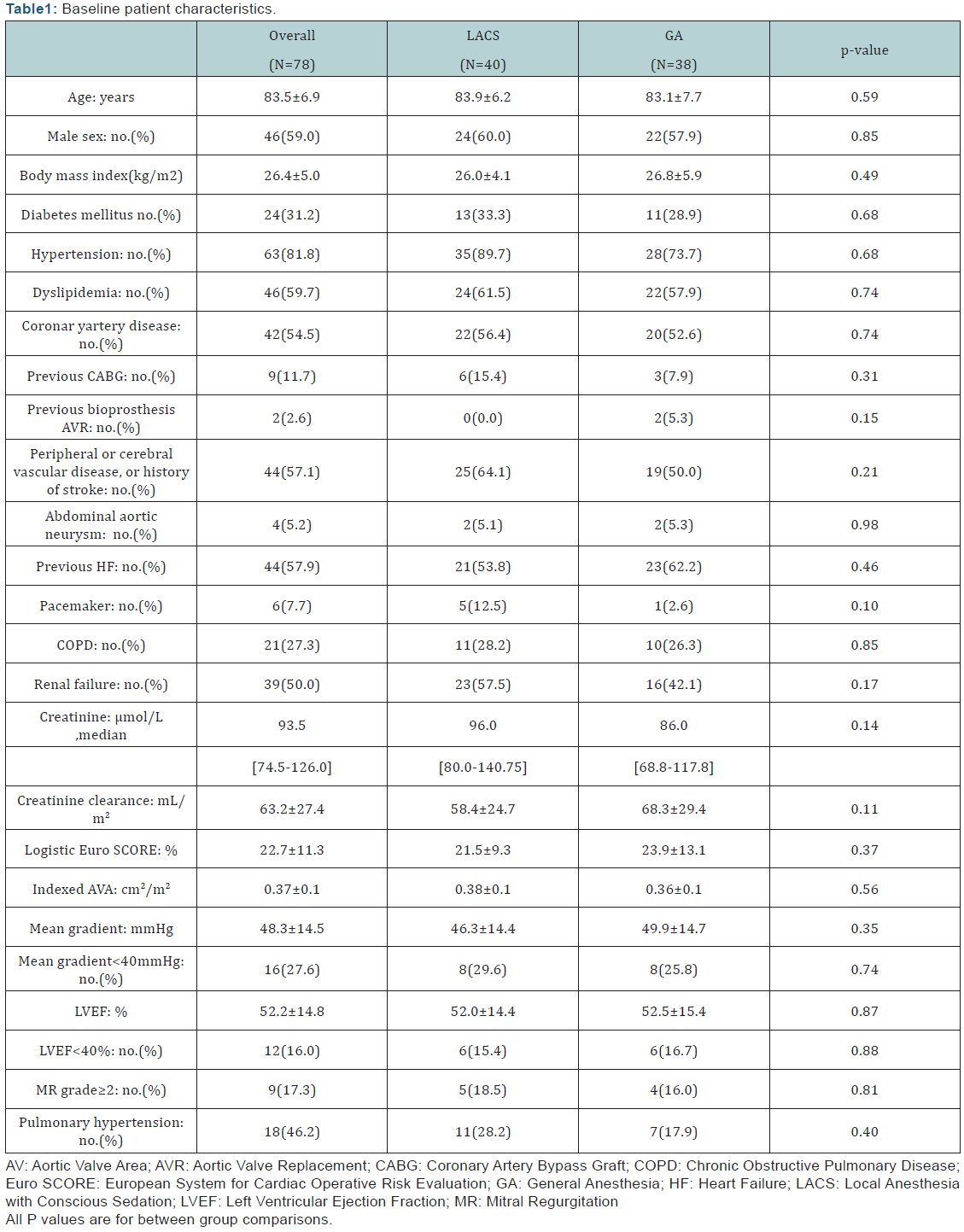
(n=8) wereduetocardiovascularcauses.Thedistributionofthe30-
daymortality according to its timing related to the procedure is
illustrated in (Figure 1).
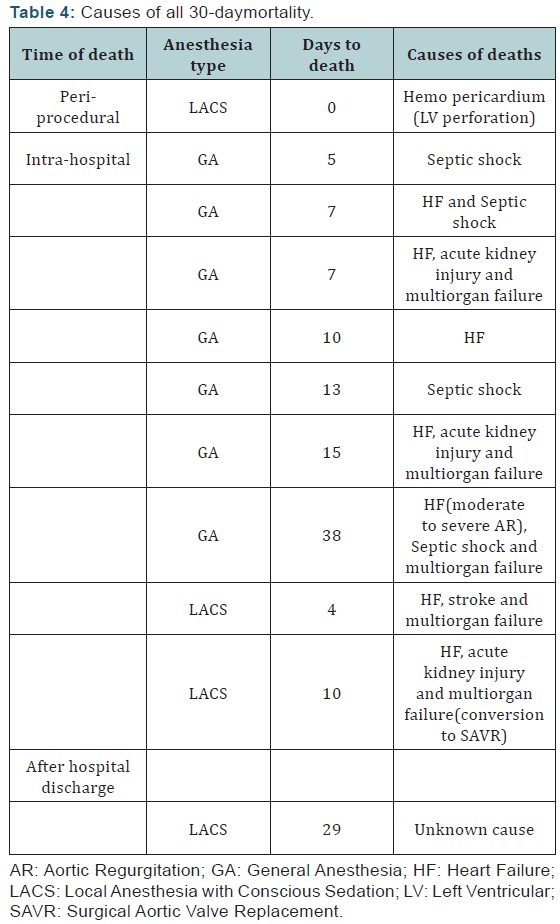
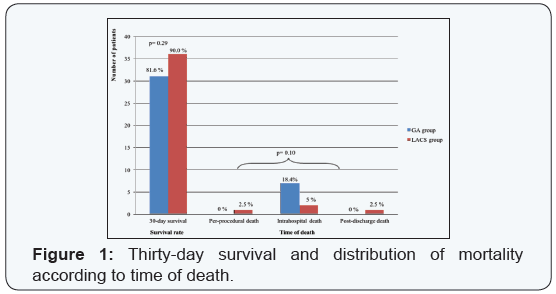
The details of all-cause 30-daymortality are shown in (Table
4). One death occurred on the day 38 post-procedure in GA
group and was included in the 30-daymortality, as it occurred
during the index hospitalization. A single peri-procedural
death occurred in LACS group, due to a perforation of the left
ventricular wall. In GA group, all 30-day deaths were intrahospital,
but none was peri-procedural. One peril-procedural
myocardial infarction occurred in LACS group, diagnosed after
the procedure. This case had conversion to on-pump SAVR. One
stroke occurred in LACS group. The prevalence of bleeding,
vascular complications and acute kidney injury was the same
between the 2 groups (Table 3). No deaths were directly related
to a vascular access site complication.
Regarding the prosthetic valve performance, results were
similar between the 2 groups (Table 3). Overall significant
prosthetic aortic valve regurgitations (grade≥2), occurred in14
of 73 patients (19.2%), without significant difference between
the two groups (p=0.78).
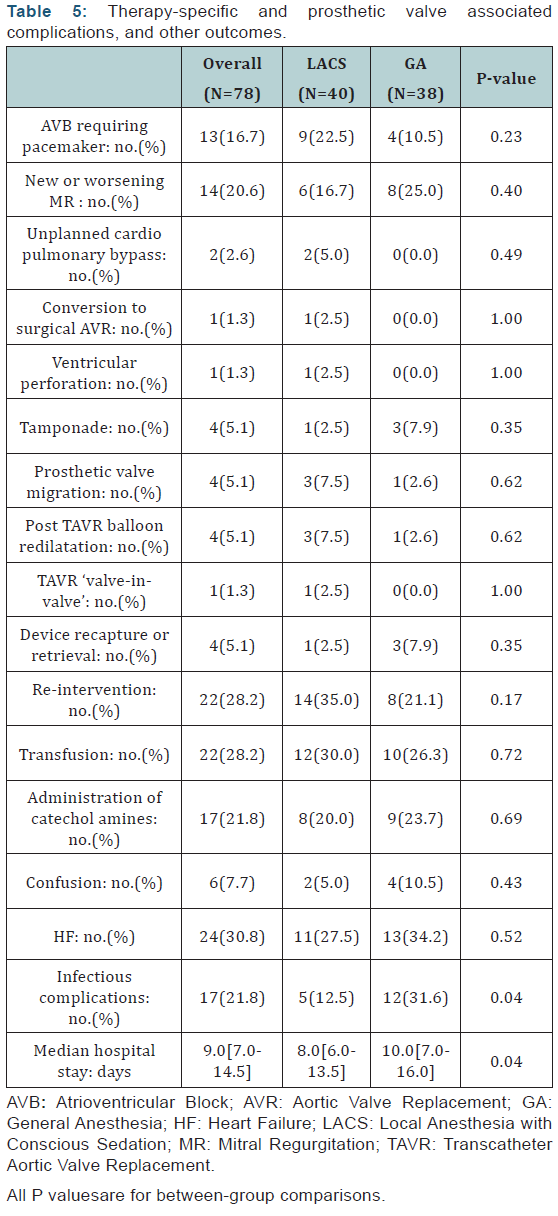
Prosthetic valve ‘associated’ complications and therapyspecific
complications were similar between LACS and GA groups
(Table 5). The occurrence of Atrioventricular bloc requiring a
permanent pacemaker implantation didn’t differ significantly
between the two groups. Conversion to an ‘open’ SAVR was
performed in1case in LACS group. Requirement of any cardiac or
vascular surgery after the index procedure was similar between the 2 groups, in 14(35.0%) patients in LACS group and 8 patients
(21.1%) in GA group, p=0.17. Reasons for those subsequent
surgeries were in LACS group: pacemaker in 9 patients, migration
of the bio prosthesis in 1case, trans apical TAVR after failure of
the femoral access in 1case, surgical repair of left ventricular
wall ruptures in1case, insertion of extracorporeal membrane
oxygenation in1case and wound infection of the scarp in1case.
In GA group indications of re-interventions were: pacemaker
implantation in 4cases, pseudoaneurysm in1case, surgical
drainage of pericardial effusion in 2cases and wound infection
of the scarp in1case.
Some anesthesia and intensive care related aspects are
also presented in (Table 5). Infectious complications rate was
significantly lower in patients receiving LACS compared to those
receiving GA (5patients [12.5%] vs.12 patients [31.6%],p=0.04).
Bronchopulmonary infections were the predominant infectious
complications in GA group (8 cases of 12). In univariate analysis,
we found that a history of chronic obstructive pulmonary disease
was associated with the occurrence of bronchopulmonary
infection (p=0.019) in GA group, where as this association was
not found in the LACS group (p=1.00). The median of hospital
stays was shorter in the LACS group (p=0.04).
The choice of anesthesia type for TF-TAVR is still controversial.
In FRANCE 2 registry TAVR were performed with the use of local
anesthesia for 40.8% of TAVR with femoral approach [11]. In the
subanalysis of the French aortic national Core valve and Edwards
2 registry that compared clinical outcomes and safety of TAVR
under GA versus local anesthesia, no benefit of LACS over GA was
demonstrated. But the lack of finding any benefit could be due to
the nationwide nature of the registry, some bias could be due
to the self-report of results individually by each participating
center. The aim of this study was to analyze and compare early
outcomes of F-TAVR between patients consecutively undergoing
LACS versus GA groups. This study has the advantage of being
well balanced in terms of the size of each group (LACS and GA
groups), in contrast to other similar studies [12,13].
Our results suggest that TF-TAVR is feasible under LACS as
well as it is under GA. The 2 groups were equivalent in terms of
device success rate and prosthetic valve performance, without
any increase in prosthetic valve associated complications or
therapy-specific complications in LACS group at 30-day follow
up (Tables 2,3,5). The distribution of all-cause 30-daymortality
depend in go the time of death (Figure1) did not differ
significantly between the 2 groups (p=0.10).
The previously reported studies considered that the TEE
was useful for the correct positioning of the prosthesis [12]. In
our study, in principle the patients in the LACS group didn’t have
TEE and it was used in all the patients in the GA group. Thus,
some of the expected disadvantages of the LACS were related
to the lack of the use of TEE and its potential consequences on the accuracy of the bio prosthesis positioning. But nevertheless
prosthetic valve performance in LACS group was not less good
than in the GA group (Table3).
TAVR under LACS seems to be as safe and effective as TAVR
under GA. GA certainly allows more comfortable working for the
interventional team compared to the LACS by facilitating the
procedure as the patient is completely relaxed and motionless.
In our experience the use of LACS wasn’t associated to any
increase of vascular complications or peril-procedural bleeding
complications (Table 3).
Were ported also in this study severe surgical complications
of TF-TAVR, and their rate seemed to below.
In the present study, the 2groups were equivalent in terms of
the use of catecholamine’s (Table 5); unlike others studies where
the use of catecholamine’s was higher in the GA group compared
to the LACS group [12]. Our result could be explained by our
method of induction of GA, which was slowly and carefully
titrated to achieve the targeted effect. Post-procedural confusion
was not less frequent as expected in LACS patients (Table 5).
This similarity between the two groups might be due to the use
of sedative agents and opioids even at low doses in LACS group.
Study limitations
This study reflects single-center experience, with limited
number of patients. The study was observation a land nonrandomized,
so we can’t conclude that the differences observed
between TF-TAVR under LACS versus GA were due only to the
type of anesthesia used. GA was used mainly during the initial
phase of the study and was shifted progressively to LACS, so we
can’t exclude that an increasing experience with time bias could
have an effect on the results.
This study showed that TF-TAVR is feasible under LACS
as well as it is under GA, with similar device success rate and
prosthetic valve performance between the two groups. TAVR
under LACS seems to be as safe and effective as the TAVR under
GA. Thirty-day mortality didn’t differ between the groups.
TAVR under LACS seems to be associated with a lower rate of
in factious complications and a shorter median hospital stay
compared to GA group. The LACS was considered by the heart
center’s team as the preferred type of anesthesia for patients
undergoing TF-TAVR.
Dr. Pascal Leprince is a proctor for Medtronic Core Valve and
a consultant for Edwards Life sciences.
We are grateful to Sadia Mohammedi, Clinical Research
Associate at LaPitie-Salpetriere university hospital, for her input
during end points collect.
For more articles in Open Access Journal of Cardiology & Cardiovascular
Therapy please
click on:
https://juniperpublishers.com/jocct/index.php
https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment