Post Cardiac Surgery Ventricular Electrical Storm, A Successful Management - Case Report and Discussion-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY &
CARDIOVASCULAR THERAPY
Background: ventricular
electrical storm is not so uncommon these days. It is a life threatening
complication in post operative period which physicians have to face
sometimes during practice. Management of electrical storm is very
complex and challenging. Ventricular electrical storm can be of three
types- Monomorphic Ventricular tachycardia, Polymorphic ventricular
tachycardia and Ventricular fibrillation.
Case report: This was an
8-year-old boy admitted for redo- aortic valve replacement. After
surgery in immediate post operative period in ICU he developed severe
ventricular electrical storm. This was a polymorphic ventricular
tachycardia following aortic valve replacement surgery. This patient
received 45 defibrillatory shocks and other anti-arrhythmic medicines
and then full sedation and paralyzing agents to revert to normal sinus
rhythm. Outcome was very satisfactory with normal sinus rhythm and no
residual neurological deficit or any other abnormality.
Conclusion: Ventricular
electrical storm is severe life threatening complication. It needs early
detection and intervention to control the event. It can be controlled
by defibrillations and combination of multiple intravenous
anti-arrhythmic drugs.
Keywords: Ventricular electrical storm; Defibrillation
Abbreviations: ES: Electrical
Storm; VT: Ventricular Tachycardia; ECG: Electrocardiogram; LVEF: Left
Ventricular Ejection Fraction; ABG: Arterial Blood Gas; ACLS: Advanced
Cardiac Life Support
Introduction
TElectrical storm is not so uncommon these days and
it’s a life threatening complication in cardiac surgery. Electrical
storm has been infrequently reported in children. The term electrical
storm (ES) was introduced in the 1990s to describe a state of electrical
instability of the heart characterized by a series of malignant
ventricular arrhythmias in a short period of time [1]. Electrical storm
is defined as the recurrence of hemodynamically unstable ventricular
tachycardia and/or ventricular fibrillation, twice or more in 24 hours,
requiring electrical cardioversion or defibrillation [2]. With the
arrival of the implantable cardioverter defibrillator, this definition
was broadened, and electrical storm is now defined as the occurrence of 3
or more sustained episodes of ventricular tachycardia, ventricular
fibrillation, or appropriate shocks from an implantable
cardioverter-defibrillator within 24 hours. Sustained VT lasts 30
seconds, involves hemodynamic compromise, or requires intervention to
terminate the episode. The episodes of VT
must be separate, meaning that the persistence of ventricular
tachycardia following inefficacious intervention is not regarded as a
second episode [3]. By contrast, a sustained ventricular tachycardia
that resumes immediately after (≥1 sinus cycle and within 5 minutes)
efficacious therapeutic intervention by the defibrillator is regarded as
a severe form of electrical storm [3].
This condition has been described in patients with
post-infarction ischemic heart disease, various forms of cardiomyopathy,
valve disease, corrected congenital heart disease and genetically
determined heart diseases with no apparent structural alteration, as for
example in Brugada syndrome [4]. The mechanisms of electrical storm are
quite complex and not well understood. Each case of electrical storm
may represent different underlying cause and electrophysiologic
mechanism. It has been postulated that cellular and molecular
alterations can increase intracellular calcium overload and changes of
the action potential duration and morphology that lead to the onset of
electrical storm [5]. Effective management of electrical storm needs
good knowledge of mechanism of electrical storm, available treatment
options, and ICD and
emergency techniques to treat refractory cases.
Case Report
Here we are presenting a case of an 8-year-old-boy, who
was admitted to our hospital with complaints of progressive
breathlessness and intermittent fever for one month. He was
operated 1 year before for congenital aortic stenosis and
had aortic valve replacement done with 21 mm sized St. Jude
Bio-prosthetic valve. He was treated for suspected infective
endocarditis in another hospital. After admission to our hospital
proer examination and full investigations were done.
- Electrocardiogram (ECG): Preoperative 12-lead electrocardiogram (ECG) showed sinus tachycardia with normal QTc interval (0.42 sec) and left bundle branch block pattern.
- Echocardiography: A 2D echocardiography with color doppler study showed severe left ventricular dysfunction. Preoperative left ventricular ejection fraction (LVEF) was 25% only, with stuck aortic valve (no vegetations).
- Laboratory investigations: Full septic screening was sent to rule out infective organism. His initial blood cultures were negative. He developed hemodynamically stable ventricular tachy-cardia after admission and was started on amiodarone Intravenous infusion.
- Surgery: He underwent repeat aortic valve replacement with 19 mm sized TTK Chitra aortic mechanical tilting disc prosthesis. Intraoperative findings revealed stuck aortic valve with vegetations; valve tissue was sent for histological and microbiological study and it came as carbapenem resistant Klebsiella pneumoniae.
Post operative period
He was on ionotropic and ventilator supports in ICU. He had
sinus bradycardia. Amiodarone was tapered over 36 hours and he
was maintained on overdrive AV sequential pacing. The patient
was in low cardiac output state with fluctuating hemodynamics
and blood pressure was maintained with adjusting inotropic
support. Echocardiography was done in first post-operative
day. Postoperative trans-esophageal echocardiography revealed
biventricular dysfunction (LVEF 10-15%), and no residual
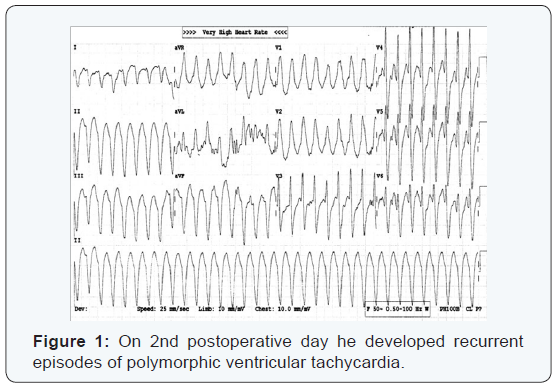
gradient across aortic valve. On 2nd postoperative day he
developed recurrent episodes of polymorphic ventricular
tachycardia (Figure 1). Arterial blood gas (ABG) analysis showed
normal electrolytes and acid base physiology. In next 8 hours, 45
DC shocks (up to 8J/kg) were delivered due to recurrence of VTs
after transient reversion to sinus rhythm. He also received two
boluses of intravenous (IV) amiodarone (5 mg/kg) and repeated
doses of IV lidocaine (1 mg/kg) followed by their infusions.
Since the patient was poorly responsive, he was also started on
IV esmolol infusion after bolus.
There was no significant change in QTc interval despite
multiple doses of amiodarone. Magnesium sulphate was given
and electrolytes corrected. Finally, it was controlled with deep
sedation and paralysis with fentanyl, midazolam and vecuronium,
with infusions of lidocaine at 40 μg/kg/min, amiodarone at 20
μg/kg/min and esmolol at 100 μg/kg/min. Post-event, he had
LVEF of 10% with septal and apical akinesis, borderline low
blood pressure and high left atrial pressure. Inotropic support
was reoptimized with dobutamine and milrinone, and ventilation
was continued for next 72 hours. His left ventricular function
gradually improved and he was extubated on 6th postoperative
day with normal neurological status. He was continued on oral
amiodarone, metoprolol and acetyltolinesterase inhibitors.
- Follow up: At follow-up 14 days later, he was in sinus rhythm consistently.
Discussion
Incidence
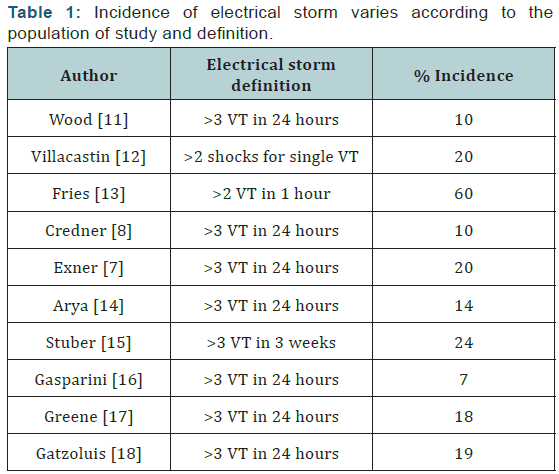
Incidence of electrical storm varies according to the
population of study and definition (Table 1) In a MADIT-II substudy of 719 patients [6], 4% developed electrical storm
over an average of 20.6 months. Electrical storm might be an
independent risk factor for cardiac death. In the AVID trial [7]
patients with electrical storm had an increased risk of nonsudden
cardiac death (risk ratio, 2.4). In the Madit -II substudy,
patients with electrical storm had a 7.4-fold higher risk of death
than patients without electrical storm6. Both studies showed
that the risk of death was highest within the first 3 months
after a storm. The prognosis remained poor for patients who
survived the initial period of electrical instability. It is unclear
whether electrical storm contributes directly to a poor outcome
or is simply a result of advanced structural heart disease [8].
Recurrent VT or VF and ICD shocks may cause left ventricular
(LV) systolic dysfunction and myocardial injury [9] which can
lead to adrenergic neurohormonal activation and exacerbate
heart failure [10].
Clinical Syndromes of Electrical Storm
Electrical storm can initially be classified on the basis of
3 gross electrocardiographic (ECG) surface morphologies:
monomorphic VT, polymorphic VT, or VF.
Monomorphic ventricular tachycardia
Monomorphic VT occurs when the ventricular activation
sequence is the same without any variation in the QRS complexes.
Most monomorphic VT is due to electrical wavefront reentry
around a fixed anatomic barrier which is most commonly scar
tissue after MI. Monomorphic VT due to wavefront reentry does
not require active ischemia as a trigger and it is uncommon in
patients who have an acute MI [11-18].
Polymorphic ventricular tachycardia
Polymorphic VT occurs when the ventricular activation
sequence on ECG consists of beat to- beat variations in the QRS
complexes. For polymorphic complexes, multiple wavefronts
must propagate throughout the heart or appear simultaneously
in several parts of the heart [19]. Polymorphic VT can be
associated with a normal or a prolonged QT interval in sinus
rhythm. Polymorphic VT is most commonly associated with
acute ischemic syndromes but can also be seen in organic heart
disease, acute myocarditis or hypertrophic cardiomyopathy.
Ventricular Fibrillation
Ventricular fibrillation is usually fatal if it is not treated
promptly. Even with defibrillation, VF may recur repeatedly and
present as electrical storm. When this happens, mortality rates
are between 85% and 97% [20]. Ischemia, which is the primary
mechanism of VF storm, should be the focus of treatment.
Mechanism of Ventricular electrical storm
The mechanisms of electrical storm are quite complex
and not well understood. It has been postulated that cellular
and membrane alterations can increase intracellular calcium
overload, with altered action potential duration and morphology leading to its onset [21]. The important role of increased
sympathetic tone has been well documented. Many conditions
including ischemia, surgery [22] and hyperthermia [23] can
precipitate increased adrenergic output.
Pharmacologic Therapy for Electrical Storm
Adrenergic blockade: Epinephrin and vasopressin are
recommended for pulseless VT and VF according to current
guidelines for advanced cardiac life support. Studies have shown
improved coronary blood flow and short-term survival after the
administration of epinephrine [24], but Epinephrine makes the
patient more susceptible to VF due to contribution to myocardial
dysfunction [25].
β-Blockers: β-Blockers decrease the susceptibility for VT and
VF. Although most of the β-Blockers are effective in decreasing
susceptibility but most of the studies are done with Propranolol.
The lipophilic nature of propranolol enables active penetration
of the central nervous system and the blockade of central and
prejunctional receptors in addition to peripheral β receptors
[26]. Propranolol may effectively suppress an electrical storm
even when metoprolol has failed [27]. Therefore, propranolol is
the preferred β-blocker.
Nademanee et al. [28] investigated the efficacy of sympathetic
blockade in electrical storm by comparing propranolol, esmolol,
and left stellate ganglionic blockade to combined lidocaine,
procainamide, and bretylium therapy. Their patients had
experienced a recent MI and more than 20 episodes of VT within
24 hours. Although the trial was nonrandomized, sympathetic
blockade provided a marked survival advantage (78% vs 18%
at 1wk, and 67% vs 5% at 1 yr). Despite the high doses of
propranolol, heart failure was not exacerbated. These authors
and others have suggested that the combination of amiodarone
and propranolol improves survival rates and should be the
mainstay of therapy in managing electrical storm.
In our patient, we used esmolol (predominantly a β-1
antagonist), which can be used as an infusion and dose can be
easily titrated based on response.
Amiodarone: Amiodarone is widely used in the treatment
of electrical storm [29]. In acute amiodarone therapy, rapid
intravenous administration blocks fast sodium channels, inhibits
norepinephrine release, and blocks L-type calcium channels.
Amiodarone can be effective even when other agents have been
ineffective. Levine et al. [30] examined 273 hospitalized patients
who had electrical storm that was refractory to lidocaine,
procainamide, and bretylium therapy. When amiodarone
was given, 46% of the patients survived for 24 hours without
another episode of VT, and another 12% responded after taking
amiodarone plus another agent. Current Advanced Cardiac Life
Support (ACLS) guidelines recommend amiodarone for cardiac
arrest in children associated with shock-refractory VT/VF.
Studies examining the effect of intravenous amiodarone in the
management of electrical storm have reported its efficacy [1].
Class I Antiarrhythmic (Sodium Channel-Blocking) Agents
Lidocaine binds to fast sodium channels and binding
increases under cellular conditions that are common in
ischemic VT, such as a reduced pH, a faster stimulation rate,
and a reduced membrane potential [31]. However, outside the
setting of ischemia, lidocaine has relatively weak antiarrhythmic
properties: conversion rates from VT to sinus rhythm range from
8% to 30%. If lidocaine is used, it should be administered as an
intravenous bolus of 0.5 to 0.75 mg/kg that is repeated every 5
to 10 min as needed. A continuous intravenous infusion of 1 to 4
mg/min maintains therapeutic levels. The maximum total dose
is 3 mg/kg over 1 hr. Procainamide- When given as a loading
dose of 100 mg over 5 min; procainamide is a reasonable choice
for terminating monomorphic VT. In patients with depressed
systolic function, procainamide can cause hypotension or
prolong the width of the QRS complex by more than 50%, which
would necessitate discontinuation of the drug.
Anesthetic agents
All patients who have electrical storm should be sedated.
Short-acting anesthetics such as propofol, benzodiazepines, and
some agents of general anesthesia have been associated with the
conversion and suppression of VT [32].
Non pharmacologic therapy
Mechanical assisted devices as Intra-aortic balloon pump,
extra corporeal membrane oxygenator supports, left ventricular
assisted devices can also be used as non pharmacological agents.
These devices increase coronary perfusion pressure and can
dramatically relieve the ischemic substrate.
Electrical Storm in ICD Patients
ICDs do not prevent arrhythmias and implanting an ICD
is contraindicated in the acute phase of electrical storm.
Intravenous analgesics and sedatives should be given early and
aggressively to patients who sustain multiple ICD shocks [33].
If an ICD fails to convert a life threatening rhythm, external
defibrillation pads should be ready for use. Being very unstable
nature of the disease, electric storm often requires combination
therapy. Manolis, et al. [34] reported a case using triple drug
intervention with a beta antagonist, class III antiarrhythmic, and
a class IB antiarrhythmic.
Conclusion
Ventricular electrical storm is a challenging situation.
Despite repeated defibrillations and severe left ventricular
dysfunction, our patient made a good recovery with aggressive
supportive treatment. It is advisable that clinicians should be
well versed with Pediatric Advanced Life Support guidelines to
manage these challenging resistant arrhythmias.
For more articles in Open Access Journal of Cardiology & Cardiovascular
Therapy please click on:
https://juniperpublishers.com/jocct/index.php
https://juniperpublishers.com/jocct/index.php


Comments
Post a Comment