Outcome of Paradoxical Low‐flow Low‐gradient Severe AS Following TAVI: Paradoxical vs. Parallel to Outcome of High Gradient AS-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Background:Transcatheter aortic
valve implantation (TAVI) is already a proven choice of treatment for
patients with severe aortic stenosis (AS). There is few data of the real
world regarding the echocardiographic and clinical outcome of patients
with paradoxical low‐flow low‐gradient aortic stenosis (PLFLG) after
TAVI procedure.
Objectives:In this study we aimed to
compare the echocardiographic and clinical outcome in a group of
patients with PLFLGAS after TAVI with a group of high‐gradient aortic
stenosis (HGAS), both groups with preserved left ventricle ejection
fraction (LVEF).
Methods:We studied the baseline
echocardiographic parameters and clinical data of 178 patients with
severe AS and preserved LVEF before TAVI and after one year of follow
up. All data were obtained from the clinical file and the workstation in
the echo‐lab of our hospital. Patients with low ejection fraction and
incomplete data were excluded. Patients were divided into two groups as
following: Group I PLFLGAS n=32 (18%); Group II: HGAS n=146 (82%).
Results:Baseline characteristics in
both groups were similar. Mean age was group I: 82±5.4 and II:
83±5.9yrs. LVEF I: 61±6.8% and II: 64±8.2%. Mean gradient I: 29±5.2mmHg
and II53±12.8mmHg (p=0.001). Global longitudinal strain (GLS) I:
‐15.1±2.5% and II: ‐16.3±4.1%. Valvulo‐arterial impedance (Zva) I:
5.7±0.9 and II 5.3±13 (p= 0.07). At one year no statistically
significant differences were found between groups. LVEF I: 64±8.3% and
II: 65±7.9%. Mean gradient I: 7.2±3.7mmHg and II: 8.3±3.9mmHg. GLS:
‐17.85%±3.8% and II: ‐17.5±5.5%. NYHA class: Group I: 2 and II: 1
(p=0.037). Regarding NYHA class there were no significant differences at
baseline and at follow up, patients in‐group I showed better NYHA class
I.
Conclusion:In this real world
sample, the clinical and echocardiographic outcome of patients with
PLFLGAS is similar to that of patients with HGAS. There is lack of
information regarding the specific cause of low flow‐low gradient in
these patients, but apparently and at least in this series has no impact
on the outcome.
Abbreviations: TAVI: Transcatheter Aortic valve Implantation; PLFLGAS: Paradoxical Low‐Flow low‐gradient Aortic Stenosis; HGAS: High‐Gradient Aortic Stenosis; LVEF: Left Ventricle Ejection Fraction; GLS: Global Longitudinal Strain; SVI: Stroke Volume Index; Zva: Valvulo‐Arterial Impedance; NYHA: New York Heart Association; CCSA: Canadian Cardiovascular Society Grading of Angina Pectoris
Introduction
The progressive aging of the population in developed
countries and the widespread of preventive screening programs of
patients with cardiovascular risks result in an increasing number of
patients diagnosed with significant aortic stenosis who are candidate
for transcatheter aortic valve implantation.
In recent years, in clinically suitable patients for
TAVI the role of cardiovascular imaging is critical in the assessment of
candidates for TAVI, providing both anatomic and hemodynamic
information. These modalities of cardiac imaging assist in choosing the
best interventional approaches and the prosthetic valve type and its
accurate sizing. According to the current ESC
and ACC/AHA guidelines, severe AS is defined by an AVA of
<1cm2 (<0.6 cm2/m2), a peak transvalvular velocity of 4 m/s, a
mean aortic valve gradient of >40 mmHg.
But beside the first main group (high gradient with normal
ejection fraction ≥ 50%) there are two groups where the
diagnosis of severe AS may be challenging and should be noted:
the second group: low flow low gradient severe AS with the
presence of LV systolic dysfunction. These modalities of cardiac
imaging assist in choosing the best interventional approaches
and the prosthetic valve type and its accurate sizing. According
to the current ESC and ACC/AHA guidelines, severe AS is defined
by an AVA of <1cm2 (<0.6 cm2/m2), a peak transvalvular velocity
of 4 m/s, a mean aortic valve gradient of >40 mmHg. But beside
the first main group (high gradient with normal ejection fraction
≥ 50%) there are two groups where the diagnosis of severe AS
may be challenging and should be noted: the second group: low
flow low gradient severe AS with the presence of LV systolic
dysfunction.
In this case, Dobutamine stress echocardiography has been
shown to distinguish between true severe and pseudo‐severe AS
and provide useful information concern in contractile reserve. A
third group consists of patients with paradoxical low flow low
gradient severe AS. In this group, left ventricular (LV) ejection
fraction is well preserved. Although transcatheter aortic valve
implantation (TAVI) is already a proven choice of treatment
for patients with severe aortic stenosis (AS), there is few data
of the real world regarding the echocardiographic and clinical
outcome of patients with paradoxical low‐flow low‐gradient
aortic stenosis (PLFLG) after TAVI procedure.
In this study, our aim was to compare the echocardiographic
and clinical outcome in a group of patients with PLFLGAS after
TAVI with a group of high‐gradient aortic stenosis (HGAS), both
groups with preserved left ventricle ejection fraction (LVEF).
For this prospective cohort study, we included only our
patients who had evidence of severe AS and a preserved LVEF
and were candidates for TAVI intervention after the informed
consent had been provided for them and the study protocol had
been approved by the ethical committee of research foundation
in the hospital. These patients were subdivided into 2 groups
depending on whether they had normal LV flow output (high
gradient severe AS with normal ejection fraction) or paradoxical
low flow output (paradoxical low flow low gradient severe
AS). We studied the baseline echocardiographic parameters
and clinical data of 178 patients with severe AS and preserved
LVEF before TAVI and after one year of follow up. All data
were obtained from the clinical file and the workstation in the
echocardiography‐lab (Q‐ lab Advanced Quantification software,
Philips Ultrasound, USA) at our hospital. Patients with low LV ejection fraction and patients with normal flow‐under gradient
were excluded.
A. Clinical data:Included history of smoking, documented
diagnosis of hypertension, hypercholesterolemia, diabetes,
obesity, coronary heart disease and previous myocardial
infarction beside symptoms (angina, heart failure, syncope)
and the quality of life. These data were collected in all
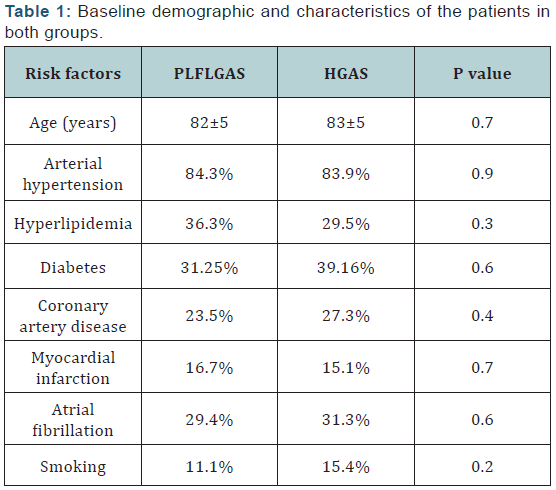
patients. All risk factors are described in (Table 1).
B. Echocardiographic data:LV ejection fraction was
measured with use of Simpson biplane method and 3D echo.
Stroke volume was measured by pulsed wave Doppler in the
LV outflow tract and was indexed for body surface area, AVA
using the continuity equation and transvalvular gradients
using the modified Bernoulli equation. We paid particular
attention to search for the highest peak transvalvular velocity
with the use of multi‐window continuous‐ wave Doppler
interrogation. 2D and 3D TTE and TEE were used to confirm
the valve stenosis severity and calculate effective orifice
area and aortic valve regurgitation during the intervention
and the follow up studies. Values of peak global longitudinal
strain (GLS) and peak segmental longitudinal strain (SLS)
values obtained by Speckle‐tracking echocardiography
(STE) in (Q‐lab Advanced Quantification software, Philips
Ultrasound, USA). As a measure of global LV after load, we
calculated the valvulo‐arterial impedance by dividing the
sum of systolic blood pressure and mean transvalvular
gradient by the stroke volume index.
C. Statistical analyses:Continuous variables were
expressed as the means and standard deviations; categorical
variables were expressed as proportions. The student t‐test
was used to test for the differences in normally distributed
continuous variables, and the Wilcoxon rank sum test was
used for comparisons involving the variables that were not
normally distributed. Categorical variables were compared with the χ2 test or Fisher exact test as appropriate. A twosided
p‐ value of less than 0.05 was considered to represent
a statistically significant difference.
From the entire cohort study, 60% of 178 patients were
women. Age and gender distribution was similar between the
two groups. Group I PLFLGAS n=32 (18%) and Group II: HGAS
n=146 (82%). Mean age was group I: 82±5.4 yrs and II: 83±5.9
yrs. Euroscore: Group I: 14.49±5.9 and Group II: 17.35±8.7
(P=0.027). Prophetic valves (58 Medtronic Core Valve 32%
and 120 Edwards Sapien 68%) had been implanted with no big
difference between both groups.
a) Pre TAVI procedure data:Baseline characteristics
in both groups were similar. Body surface area was similar
in both groups (I: 1.64±0.15 kg/m² and II: 1.60±0.1 kg/m²,
P=0.18). The values of systolic blood pressure were in the
range between 130‐160 mmHg (P=0.9) and the heat rate in
the range 60‐80 bps. The values of LVEF (I: 61±6.8% and
II: 64±8.2%) and GLS (I: ‐15.1±2.5% and II: ‐16.3±4.1%)
were similar in both groups. The stroke volume and flow
rate were lower in group I patients than in group II, (SVi:
I: 24.01 ml/m² and II: 28.16 ml/m²) (P= 0.09) and patients
in group I also had smaller LV end‐diastolic volume index
(LVEDVi group I: 47.15±13.3 ml/m² and II: 50.05±14.5 ml/ m²). Patients in group I had lower gradients despite a similar
AVA and indexed AVA compared with patients in the group II
(Mean gradient I: 29±5.2mmHg and II: 53±12.8mmHg) (p=
<0.001). The valvulo‐arterial impedance was higher in group
I patients (Zva: 5.7±0.9 mmHg/mL m²) than in group II (Zva:
5.3±13 mmHg/mL m²) (p= 0.07). The majority of patients
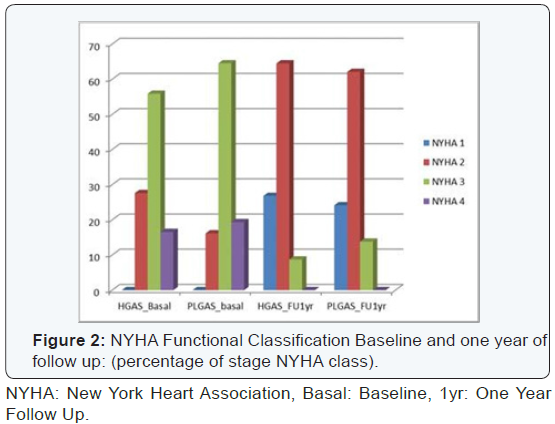
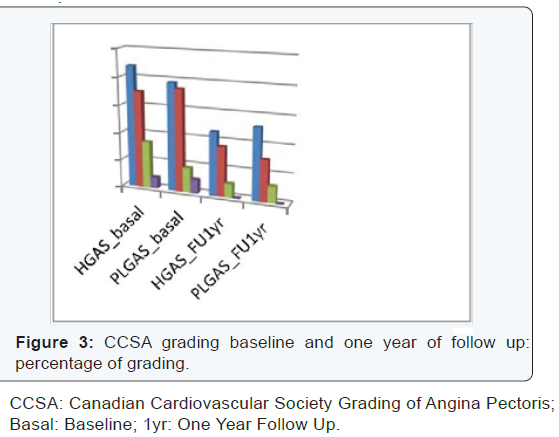
were symptomatic (NYHA class 2 to 4) (CCSA class 1 to 3).
b) Follow up data:At one year no statistically significant
differences were found between both groups. LVEF (I:
64±8.3% and II: 65±7.9%). Mean gradient (I: 7.2±3.7mmHg
and II: 8.3±3.9mmHg). GLS: (I: ‐ 17.85%±3.8% and II:
‐17.5±5.5%). Indexed Aortic prosthetic valve effective orifice
area (EOA) index: (EOA index I: 1.66±0.3 cm2/m2 and II:
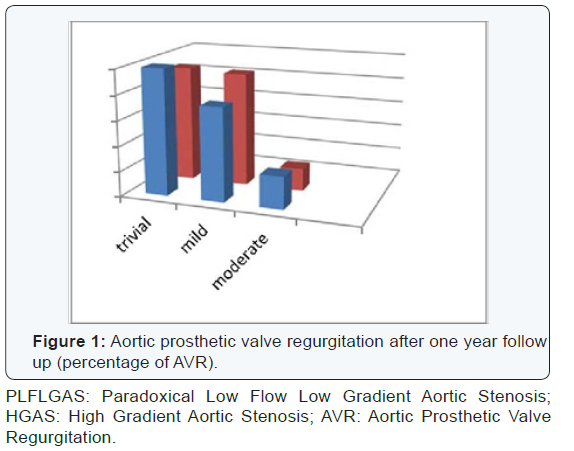
1.44±04 cm2/m2). Aortic prosthetic valve regurgitation
(AVR I; trivial: 50%, mild: 37%, moderate: 13% and AVR
II; trivial: 46%, mild: 45%, moderate: 9%). Regarding
prosthesis/patient mismatch (VP-PM); 35% of patients
showed mild VP-PM with no big difference between both
groups and both types of prosthetic valve. One year global
mortality was similar in both groups (group I: 10% and
II: 12%, P= 0.9). Both groups showed better CCSA class at
follow up. Regarding NYHA class there were no significant
differences at follow up; patient’s in‐group I showed better
NYHA class I. The study results are described in (Table 2)
and (Figures 1-3).
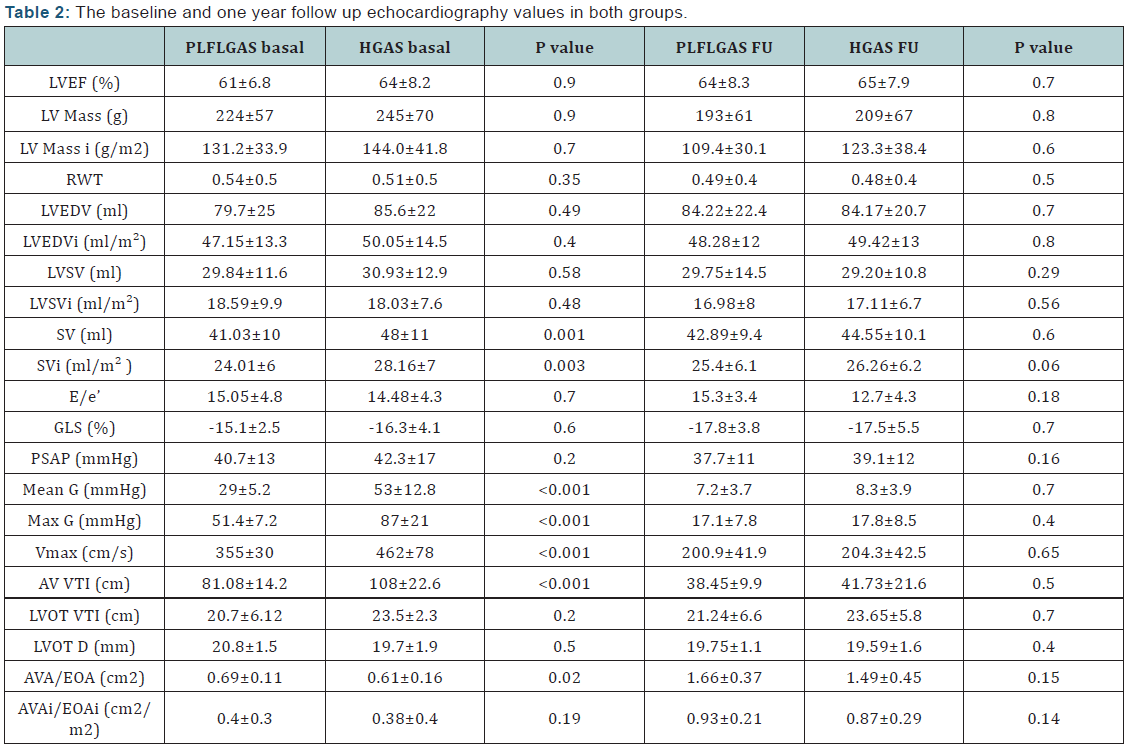
FU: Follow Up; I: Index; D: Diameter; G: Gradient; PLFLGAS:
Paradoxical Low-Flow Low-Gradient Aortic Stenosis; HGAS: High
Gradient Aortic Stenosis; LVEF: Left Ventricle Ejection Fraction;
LVEDV: Left Ventricle End Diastolic Volume; LVSV: Left Ventricle
Systolic Volume; SV: Stroke Volume; RWT: Relative Wall Thickness;
GLS: Global Longitudinal Strain; E/e′ ratio: mitral inflow E- wave
divided by annular tissue e′ Wave; SPAP: Systolic Pulmonary Artery
Pressure; Vmax : Maximum Velocity; VTI: Velocity Time Integral;
LVOT: Left Ventricle Outflow Tract; AVA: Aortic Valve Area; EOA:
Effective Orifice Area
In this work, we showed that PLFLGAS presented the typical
Doppler echocardiographic features reported in previous
published studies [1-3] including small LV cavity size and
increased LV global hemodynamic load as reflected by high
valvulo‐arterial impedance [4]. But in contrast to studies [5,6]
revealed those patients with PLFLGAS had more myocardial
fibrosis and a markedly reduced LV longitudinal systolic function
which contribute to the reduced LV outflow and transvalvular
gradient and to the worse outcomes in these patients, our study
showed the reduced baselines values of GLS in both groups were
detected with no significant difference and the patients with
PLFLGAS showed improvement in values of global longitudinal
strain similar to patients with HGAS after one year follow up.
Although several studies reported patients with PLFLGAS,
compared to patients with HGAS, had worse symptomatic status
and prognosis after aortic valve replacement [7-9], we found
after one year follow up, compared to the basal evaluation, all
echocardiography parameters showed improvement in values
of LV structure and the functional capacity of the patients
and ultimately clinical outcome similar to HGAS patients.
Furthermore, with regard to one paper [9] that have pointed
to the influence of body mass index on the gradient across the
valve and may lead to underestimation of stroke volume index,
the majority of PLFLGAS patients had the body mass index in
the normal range. Based on these results and in respect of
studies which had shown the patients with PLFLGAS have worse
symptomatic status, our prospective study, however, showed
these patients have feature similar to that of patients with HGAS
after one year follow up, compared to the basal evaluation, like
previous published studies [4,10] and a new published study
[11] which had reported the mid‐term prognosis after TAVI
procedure in PLFLGAS patients is similar to HGAS patients
despite higher preoperative mortality.
In addition to the above, respecting to recent data [12-14]
that confirmed lower values of indexed LV stroke volume are
independently associated with increased mortality following
TAVI, our study showed approximately 60% of patients in group
II had low SVI (35 ml/ m²) with high mean gradient (≥40
mm Hg), that makes regarding not only myocardial contractile
reserve in TAVI risk algorithms, but also LV stroke volume
reserve in HGAS with low SVI as well as in PLFLGAS would
probably be more appropriate and more clinical useful [15].
Although Dobutamine stress echocardiography should not be
used in these groups of patients, estimation of LV stroke volume
reserve, beyond the gradient across the aortic valve, should be
considered in therapeutic decision‐making of this challenging
subset of patients. Finally, we emphasize paradoxical low flow
low gradients aortic stenosis is still a challenging clinical entity
that requires special attention and careful approach including assessment of hypertension and stenosis severity and raised
many questions if this aortic‐incompetence population would
be a good target for TAVI in this time when the new generation
valves become available.
- It is a small cohort of patients referred for TAVI in a single center.
- The relation between myocardial contractile, stroke volume and gradient across aortic valve is not fully understood and studying their impact on TAVI outcomes require further research about their mismatch in big group of AS disease.
- It is a mid‐term single center study, long‐term multicenter studies are needed to evaluate TAVI effectiveness and outcome in patients with PLFLGAS.
In this real world sample, the clinical and echocardiographic
outcome of patients with PLFLGAS is similar to that of patients
with HGAS. There is lack of information regarding the specific
cause of low flow‐low gradient in these patients, but apparently
and at least in this series has no impact on the outcome.
This work was supported by a grant of 2015 European
society of cardiology training grants program.
For more articles in Open Access Journal of Cardiology & Cardiovascular
Therapy please
click on:
https://juniperpublishers.com/jocct/index.php
https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment