Medium term Follow up Treatment of Severe Native Coarctation of Aorta Using of Balloon Angioplasty in Young Infants Less Than one Year’s age-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF CARDIOLOGY & CARDIOVASCULAR THERAPY
Abstract
Background: The spectrum of
therapeutic approaches for the treatment of native aortic coarctation
has widely expanded from surgical correction to balloon angioplasty (BA)
and stent implantation. The aim of this study to assess the safety and
efficacy of BA for native CoA therapy in infants less than one year’
sold age.
Method: Sixteen patients (10
male) with discrete COA underwent BA of COA between May 2014 and May
2015 at our center. The age ranged from 23 days to 10 months (mean 4.28 ±
2.84 m) and body weight ranged from 3 to 7 kg (mean 4.76 ± 1.33 kg).
Appropriate balloons (mean 6.18± 0.91 mm) were choosedand were inflated
2-3 times under fluoroscopic guidance. Successful outcome was defined as
peak systolic pressure gradient after balloon angioplasty < 20 mm Hg
or decreased by more than 50% and at least 50% increase in diameter.
Follow-up duration was 6.0 ± 3.0 months (1-12 months).
Result: The mean value of the
peak‑to‑peak systolic pressure gradient between ascending to descending
aorta significantly decreased from 48.43 ± 11.65 mmHg (range 25-65 mmHg)
to 11.43 ± 8.29 mmHg (range 0-30 mmHg) (P < 0.001).
Echocardiographic peak and mean pressure gradients decreased
significantly from 58.81 ± 11.15 and 30.56 ± 6.51 before of procedure to
23.06 ± 11.75 and 12.31 ± 6.86 mmHg during follow-up respectively
(P<0.001).
Conclusion: For native discrete
aortic coarctation in young infants <12 months of age percutaneous BA
is a safe and effective treatment alternative to surgical approach.
Abbreviations: BA: Balloon Angioplasty; COA: Coarctation of Aorta; TTE: Transthoracic Echocardiography; ECG: Electrocardiogram; VSD: Ventricular Septal Defect; CGDRC: Child Growth and developmental Research Center
Introduction
Coarctation of aorta (COA) is a common congenital
cardiovascular defect that defined a stenosis or occlusion of the aorta,
usually located in the region of the ligamentum arteriosum after the
left subclavian artery origin [1]. It can be diagnosed over a wide range
of ages during neonatal to senile and with varying degrees of severity
from asymptomatic to severe heart failure. This defect accounts for
approximately four in 10,000 live births, which corresponds to 5-8% of
all congenital heart defects and may occurs as an isolation or in
association with other cardiac defects, most commonly ventricular septal
defect and bicuspid aortic valve.COA diagnosis may be missed delayed
until the patient has developed congestive heart failure, which is
common in infants, or hypertension, that is common after this time
during the life. Most often coarctation of aorta diagnosed during the
first months of age because of its symptoms. In the literature two types
of COA have been described: postductal (or adult) and preductal (or
infantile), depending on whether the coarctation segment is distal or
proximal to the ductus arteriosus, respectively [2].
The spectrum of therapeutic approaches for the treatment
of native aortic coarctation has widely expanded over the past
sixty years. For the first time by Crafoord in 1944 surgical
repair of aortic coarctation was performed. Many surgical
techniques have been used for coarctation repair, and each of
them had advantages and disadvantages. Percutaneous balloon
angioplasty is a less invasive alternative approach to surgical
technique for treatment of patients with a discrete coarctation
of aorta. It has been used for coarctation treatment since 1982
and less acceptable for safety and effectiveness in patients
with native coarctation [3]. For the treatment of choice for recoarctation
after previous surgical repair balloon angioplasty
(BA) is widely accepted among intervention a list to be in as
much as its morbidity is lower and its higher success rate in
comparison with repeat surgery [4].
It remains controversial for a primary treatment approach for
a native coarctation of aorta [5]. Balloon angioplasty technique
involves expansion of the constricted segment site that results
in rupture of the intima and injury of the media. Less favorable
outcomes have been described in patients with aortic arch
hypoplasia or long segment coarctation compared to discrete
type of coarctation. Complications after balloon angioplasty
include injury at the femoral or other percutaneous access site,
restenosis or recoil and damage of the aortic wall resulting in
aortic aneurysm formation. Balloon-expandable stents provide
an effective and safe therapy for many patients with coarctation
of aorta after early ages. Stents decrease restenosis after
procedure related to vessel recoil and diminish the incidence of
aneurysm formation and reduces the resting systolic gradient to
less than 5 mmHg [6,7].
Stent implantation initially was used only for cases that
balloon angioplasty and surgery had failed but in patients with
stent implantation several re-dilations may be required until
the patient is fully grown to adult age [7,8]. Therefore balloon
angioplasty is preferred in children less than10 year’s old age or
20 kg and in this group this technique had special difficulty and
experience for performing and successful. The purpose of this
study was to evaluate safety and efficacy mid-term follow-up
results of balloon angioplasty for treatment of native coarctation
of aorta during May 2014 to May 2015 in patients less than one
year’s old age in our center.
Sixteen patients with discrete COA underwent transcatheter
balloon angioplasty of COA between May 2014 and May 2015
at Shahid Chamran cardiovascular heart center of Esfahan
University of medical science. Ten patients were male and six
were female. The age ranged from 23 days to 10 months (mean
4.28 ± 2.84 m) and body weight ranged from 3 to 7 kg (mean
4.76 ± 1.33 kg). Before procedure, all patients were examined
clinically by pediatric cardiologist and underwent transthoracic
echocardiography (TTE), 12 lead electrocardiogram (ECG), and chest radiography. Clinically, all 16 patients presented with
ranges of heart failure and decreased ejection fraction and
cardiac function. Among these 16 patients, 6 had an isolated
CoA, including two patients with lowest age diagnosed with
dilated cardiomyopathy, mild endocardial fibroelastosis, before
being referred to our hospital.
The other 10 patients presented with other cardiac defects,
including ventricular septal defect (VSD) in 3, atrial septal defect
in 2, and patent ductus arteriosus in 3, bicuspid aortic valves
in 1 patient and tassig-bing malformation in one. For patient
with a CoA and tassig-bing malformation balloon angioplasty
was performed for increasing of ejection fraction and one week
later transferred to operating room subsequently. In patients
with small VSD only balloon angioplasty were performed and
in one patient with large VSD at first balloon angioplasty and
then surgical correction were performed. All of the procedures
were performed under deep sedation, and endovascular balloon
angioplasty was performed via a retrograde femoral artery
approach and standard catheterization technique. Arterial
pressure was monitored persistently during the procedure.
Coarctation of the aorta was defined as systolic pressure
gradient ≥ 20 mm Hg between ascending and descending aorta
or echocardiographic or angiographic evidence of COA.

A 4 or 5 (11 and 7 cm in length) French introducer sheath was
initially used and according to balloon size changed during the
procedure. A 4 French end and side holes catheter was passed
gently across the coarctation site retrogradly and the pressure
of ascending aorta and descending aorta were measured and
pressure gradient was estimated. After aortogram in lateral or
LAO position, size and position of coarctation was distinguished
and an appropriate balloon equal to aortic size at diaphragmatic
size or equal to or 1-2 mm greater than the diameter of the aortic
arch at or proximal to the level of the left subclavian artery no
larger than 3 times as narrowest size of coarctation site was
choosed. Mean sizes of balloons were (mean 6.18 ± 0.91 mm),
range from 5-8 mm. It passed across the coarctation site over
a floppy tip guide wire that inserted in the ascending aorta or
right subclavian artery. The balloon was inflated 2-3 times under
fluoroscopic guidance for each patient by second intervention
a list under the pressure recommended by the manufacturer.
If a residual waist or high pressure gradient were seen at the
coarctation zone, another 1-2 mm larger balloon was selected
to dilate the coarctation within the same protocol. After the procedure aortography was performed for more evaluation
(Figure 1).
Successful outcome was defined as peak systolic pressure
gradient after balloon angioplasty < 20 mm Hg or decreased
by more than 50% and at least 50% increase in diameter at the
coarctation segment. Anticoagulation with heparin (50-100U/
kg) was administrated after vascular access was achieved and
it was repeated during the procedure after one hour. Followup
duration was 6.0 ± 3.0 months (1-12 months). Followup
concentrated on examination with special attention to
systolic blood pressure gradient, blood pressure and regular
echocardiogram after procedure. Statistical significance was
evaluated utilizing the paired t-test for paired data such as pre
and post procedure mean values of the peak-to-peak systolic
gradient between ascending to descending aorta. A p value
< 0.05 was considered statistically significant. The interval
data are expressed as mean ± standard deviation. All the data
analyses were conducted using SPSS version 18.0.
In 3 patients two sequential balloon dilations were
performed, and in the other patient’s one balloon dilation was
performed. In two patients with ages lower than one month’s
(23 and 25 days old age) and severe coarctation and decreased
ejection fraction and heart failure, the catheterization gradient
before the procedure was approximately 60 mmHg. After
procedure, the pressure gradient decreased to 10 and 30 mm Hg,
and showed significant increasing in cardiac function. In this two
patients another procedure were performed 4 and 6 months
later because of increasing gradient and recoiling of coarctation.
In one of these patients after first procedure gradient increased
from 10 to 40 mm Hg and by second procedure pressure gradient
decreased to 5 mm Hg and did not increased during follow-up.
Another patient had increased pressure gradient and in second
time we could not passed from coarctation zone because of
severe constriction and she referred for surgical correction.
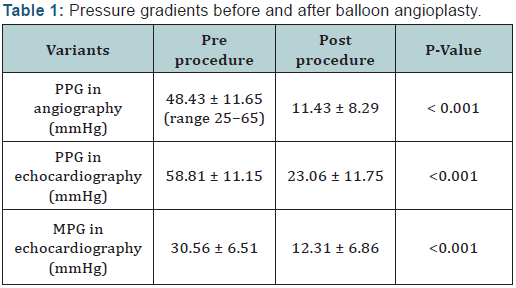
PPG: Peak Pressure Gradient; MPG: Mean Pressure Gradient
The mean value of the peak-to-peak systolic pressure
gradient between ascending to descending aorta significantly
decreased from 48.43 ± 11.65 mmHg (range 25-65 mmHg)
to 11.43 ± 8.29 mmHg (range 0-30 mmHg) (P < 0.001) at the end of procedure (Table 1). A residual gradient of greater than
20 mmHg was acceptable in the presence of an increase at
least two fold in the coarctation zone diameter. There were no
immediate complications during balloon angioplasty and need
for emergency surgery. During or post balloon angioplasty,
no angiographic complications such as retroperitoneal
hematoma secondary to bleeding from the external iliac artery,
aneurism formation, arterial dissection, aortic rupture, and
arteriovenous fistulas were evident by repeat echocardiography,
catheterization, or CT-angiography. There were no deaths
related to the procedure. None of patients has need for blood
transfusion. 3 patients had decreased femoral pulsation and by
using heparin pulses were returned normally during the first 24
hours. Regular echocardiography was performed the day after
procedure, one month, 3 months and six months later and yearly
in future. During the follow-up 1 month to 12 months follow
up period (mean 6.0 ± 3.0 months) we had not complication.
Echocariographic peak pressure gradient and mean pressure
gradient decreased significantly from 58.81 ± 11.15and 30.56 ±
6.51 before of procedure to 23.06 ± 11.75 and 12.31 ± 6.86 mm
Hg during follow-up respectively (P<0.001).
Balloon angioplasty was first used in infants and neonates
with coarctation of aorta and heart failure that were at a higher
risk for surgery than interventional approach, but its indications
were later extended to include older children, adolescents and
other ages as well [9-11]. Complications such as aneurysm
formation and re CoA have been associated with both balloon
angioplasty and surgical repair have been reported during the
past decades and it making difficult to decide any meaningful
conclusions as to which treatment option is superior to other
one. Aortic dissection and aortic aneurysm formation and aortic
rupture occurred in about less than 10% of patients at the site
of repair late after operation [12,13]. The risk of re CoA after
surgery in young children ranges from 44% to 11% in neonates
to older children, and whatever the patient had younger age the
risk is higher [14].
For the baseline probabilities of successful treatment,
aneurismal formation, re CoA and other complications, Wong
et al reviewed articles and he reported that BA was preferred
over surgery for all plausible situations as the initial treatment
for native CoA in children (15).Repeated intervention rate was
high especially in patients treated with balloon angioplasty
during infancy and younger age. The success rate immediately
after procedure in our study was 93% (15 patients), that was
concordant with other studies in which the early success rate in
infants less than 3 months ranged greater than 88% [16-19]. In
follow-up period (1m to one year) two patients developed rest
enosis and pressure gradient in echocardiography and needed a
second BA. One of these patients resolved via repeated balloon
dilation without surgery. Other patient had youngest age (23
days) among our patients and had dilated LV and critical posture at admission but after procedure her gradient was decreased
and her ejection fraction was increased and during follow-up her
gradient was increased and because of unsuccessful procedure
she was referred to the surgeon.
We guess that her condition and small balloon choosing
in first procedure occasion to re-CoA and progressing of her
gradient. Restenosis rate was reported in previous reports,
in infants younger than 12 months ranged from 25% to 71%
[14,15] and in this study we had 12.5% rest enosis among our
patients that which was lower than other studies, but longer
follow-up is need for better results. BA has the best results in
patients with developed and good size aortic arch and discrete
CoA, and in these patients further restenosis of the CoA zone can
be managed by repeat procedure. An aneurysm formation has
been reported, immediately or lately during the follow-up and it
occurs less than 6% in BA [20-22]. Aneurysms may not change
in size for a long duration of time during life and it may be not
require immediate treatment for long time. He L reported that
5.4% percent of their patients developed aneurysmal formation
and disappeared during the 18 month follow-up and they had
not any late aneurysm formation in their study [23]. We had not
any immediate aneurysmal formation among our patients and
we think that oversize balloon choosing and smaller children are
risk factors for inducing of this complication and longer duration
of follow-up is needed for further results. In patient with a
CoA and tassig-bing malformation balloon angioplasty was
performed at first and one week later transferred to operating
room subsequently with better condition.
In patients with other associated congenital heart defects
such as VSD experience and policy of that center are important
to treatment of these patients. He L reported that they used
balloon for CoA treatment and patients with large non-restricted
VSD underwent surgical repair on the same day or within 1
week [23]. We had the same policy and think that it’s better
because of low risk complications such as phrenic nerve palsy,
delayed sternal closure, and wound infection. Femoral artery
complications such as occlusion and aneurysm are the most
commonly reported artery complications in young infants
especially in younger infants and neonates [24]. Using of smaller
sheath and administering of heparin during the procedure and
short time of procedure are help to reduce these complications.
We used 4 F sheaths for beginning of procedure and during the
procedure change it with 5F short sheaths (7cm in length) if
need and if had suspicious to reduce flow to lower limb, heparin
was administrated during the first 24 h after procedure, and we
did not see these complications in our patients.
In neonatal period and severe coarctation of aorta especially
lower weight smaller balloon such as coronary balloon using
for the first time and then in follow-up redilation of coarctation
with larger balloon at older age was recommended [24]. In our
center we use smaller balloon with low profile that need smaller sheath and passing easily from CoA zone at the first time and
if need in follow-up larger balloon help us for reducing the
pressure gradient (staging procedure) and this process avoid
aneurysm formation and artery complications in smaller infants.
A limitation of our study is its small size and short term followup.
It had been better that we compared these patients with
matched group that threated with surgical approach and long
term follow-up and complications.
In conclusion, for native discrete aortic coarctation in young
infants <12 months of age percutaneous BA is a safe and effective
treatment. Smaller sheath, low profile balloon using and staging
procedure in BA are recommended for reducing complications.
However, long term close follow up is essential for these patients
that treated with BA to observe and treat late aneurysms and
restenosis. Large groups of patients and longer duration of
follow-up need for confirmation of these reports.
This study was approved and supported by Child Growth and
developmental Research Center (CGDRC), Esfahan University of
Medical Sciences, Esfahan, Iran. This paper was extracted from
the thesis of Dr. Modjdeh Gheisari.
For more articles in Open Access Journal of Cardiology & Cardiovascular
Therapy please
click on:
https://juniperpublishers.com/jocct/index.php
https://juniperpublishers.com/jocct/index.php



Comments
Post a Comment