Coenzyme Q10: The Cardiac Bio-energizer in Cardiovascular Diseases-Juniper Publishers
Juniper Publishers-Journal of Cardiology
Abstract
This systematic review is aimed to identify,
evaluate and summarize the role of oral Coenzyme Q10 supplementation in
prevention and treatment of cardiovascular diseases (CVD). CoQ10 is
concentrated primarily in the cellular mitochondria where it functions
as a co-factor transferring electrons from Complex I to Complex II, III
ultimately resulting in the formation of energy in the form of ATP.
Coenzyme Q10 an endogenous antioxidant declines in our body because of
various factors like aging, diseases and use of certain drugs like
statins, beta-blockers which exacerbate its deficiency. Deficiency of
this important endogenous antioxidant CoQ10 results in energy depleted
state. Published data and research have suggested that Coenzyme Q10 an
endogenous antioxidant has a potential for being used in the prevention
and treatment of CVDs, in particular in Heart failure and Ischemic heart
disease. Supplementation with CoQ10 not only corrects the deficiency of
CoQ10 by improving the circulating levels of CoQ10 but also shows a
significant improvement in various parameters like ejection fraction,
NYHA class, symptom score and survival rate. Being a natural substance
with low toxicity and good efficacy it would be appropriate to recommend
CoQ10 as an adjunct to conventional therapy in selected group of
patients.
Introduction
In the Western countries, cardiovascular disease
(CVD), is considered as disease of the aged. 23% of CVD death occur
below the age of 70 years, however in India a fact to worry is about the
rising incidences of CVD for people between 25-69 years, to 24.8% which
means the loss of productive population. An estimated 9.2 million
productive years of life were lost in 2000 with an expected increase to
17.9 million years in 2030 [1]. Various CVDs like angina, myocardial
infarction, heart failure and cardiomyopathies are characterized by
decreased pumping action of the heart resulting in energy deprivation
state which is usually present in the form of symptoms like fatigue and
dyspnea. However, it is thought that the risk of CVD can be reduced by
changing number of modifiable risk factors like exercise, lifestyle and
diet. Dietary supplements like Coenzyme Q10 (CoQ10), have received a
great deal of attention for the prevention of CVD as deficiency of CoQ10
is frequently seen in cardiovascular patients [2]. The clinical
experience of CoQ10 in cardiology includes studies on heart failure,
hypertension, coronary artery disease, diastolic dysfunction of the left
ventricle, also inischemia- reperfusion injury as it relates to
coronary artery bypass graft surgery (CABG) [3]. Coenzyme Q10 is a
naturally occurring fat soluble substance present in all cells of our
body with high concentration in tissues requiring high amount of energy
like the heart, liver, kidney and pancreas. Coenzyme Q10 or Ubiquinone
is a vital antioxidant present intracellularly that is synthesized by
the human body. Coenzyme Q10 plays a vital role in energy (ATP)
production in the body by acting as an electron carrier in the
mitochondrial oxidative phosphorylation and as a coenzyme for
mitochondrial enzymes [4]. 95% of the energy of the human body is
produced in the mitochondria which are required for basic functioning of
the cell like growth and maintenance. Heart, having the highest
concentration of CoQ10, utilizes it for energy dependent processes like
cardiac contraction and relaxation. It is also vital for the functioning
of various ATP regulated membrane channels as well. Besides providing
energy in the form of ATP to the heart, Coenzyme Q10 also functions as a
free radical scavenger and is potentially useful in most of the cardiac
diseases since there is increasing evidence that CVD may be associated
with energy depletion and oxidative stress resulting in increased risk
of recurrent cardiovascular events. This review gives details of
Coenzyme Q10 in various cardiovascular conditions.
Coenzyme Q10: A walk through the basic
Coenzyme Q10 was isolated in late 1950s from the
mitochondria of beef heart by a leading biochemist Dr. Fredrick
Crane. The name Coenzyme Q10 was coined after the discovery
of its structure which consisted of a quinone ring denoted by ‘Q’
along with 10-isoprenoid units in its side chain denoted by ‘10’,
and since its function was acting as a coenzyme hence the name.
It is also called “Ubiquinone” as it is present ubiquitously in all
living beings. Coenzyme Q10 being a lipid-soluble micronutrient
is endogenously synthesized in the body. Ubiquinone has a
strong influence on at least three mitochondrial enzymes
(complex I, II and III) as well as enzymes in other parts of
cell. These enzymes are involved in oxidative phosphorylation
pathway and therefore, are vital for synthesis of ATP which is
useful for various cellular functions. (Figure 1)
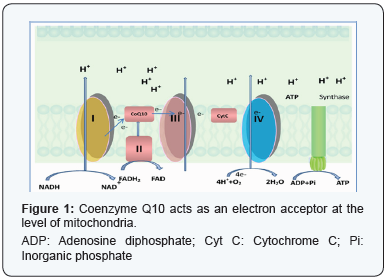
It may be useful in preventing cellular damage during
myocardial ischemia and reperfusion. CoQ10 prevents the
oxidation of lipoproteins (LDL cholesterol) and thereby inhibit
atherosclerosis and disruption of plaque. It has demonstrated
the various clinical benefits mainly due to its ability to improve
ATP production, antioxidant activity and membrane stabilizing
properties. These effects are beneficial in not only the treatment
but even prevention of cardiac disorders. The antioxidant
activity of CoQ10 confers protection against peroxidation of
lipids and works together with other antioxidants like vitamin E
in preventing the damage to plasma lipids and lipid membranes.
CoQ10 may offer significant protection against deposition of
fatty plaque (atherosclerosis) by activating smooth muscle cells
in which it is abundant, and by preventing the formation of lipid
peroxides and oxidation of LDL (low density lipoprotein). It
might have some ability to maintain the integrity of various ion
channels like sodium channels, myocardial calcium ion channels,
and potassium channels during ischemic insults [5].
Coenzyme Q10: Different forms; different functions
Coenzyme Q10 is present in all membranes throughout the
body. It is also present in the bilayered phospholipid membrane of all cells. The quinone head group of CoQ10 can be in the
oxidized (ubiquinone) or in reduced (ubiquinol) form. Most
membranes have enzyme systems that are defined to reduce
the ‘quinone (oxidized form)’ and oxidize the ‘quinol (reduced
form)’. The percentage in quinol form in various membrane
ranges from 30-90% depending upon the metabolic state of
the cell [6]. The oxidized form of CoQ10 (Ubiquinone) helps in
generation of ATP; while the reduced form of CoQ10 (Ubiquinol)
acts as an antioxidant.
Sources of Coenzyme Q10
CoQ10 is present in all tissues but is highest in the heart,
skeletal muscles, liver and kidney and lowest in the lungs. The
normal plasma CoQ10 level in a healthy adult ranges from 0.68-
1.1 μmol/L, which is maintained mainly by endogenous synthesis,
and to a lesser extent by the ingestion of foods containing CoQ10
[7]. Coenzyme Q10 can be obtained by endogenous biosynthesis
and dietary intake. Intracellular synthesis in human body is
the major but not the only source of CoQ10. The rest can be
synthesized in the liver from nourishment [8].
Endogenous Biosynthesis of CoQ10: Coenzyme Q10 is
synthesized in almost all human tissues. The biosynthesis of
Coenzyme Q10 is a 17-step complex process requiring at least
8 vitamins and several minerals. However, the 3 major steps are:
- Synthesis of benzoquinone structure from either tyrosine or phenylalanine
- Synthesis of Isoprenoid side-chain from acetyl coenzyme A (Acetyl CoA) via Mevalonate pathway
- Condensation of the above 2 structures.
One essential step regulating the synthesis of CoQ10 seems
to be the hydroxymethylglutaryl (HMG)-coenzyme A reductase
reaction, common with a step in cholesterol synthesis. (Figure 2)

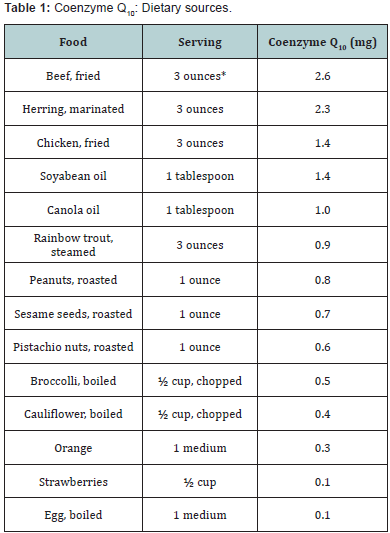
Dietary Sources: Coenzyme Q10 is found naturally in
dietary sources. It is present in a wide variety of food from
animal and vegetable sources. In animal sources, large amounts
are present in organ meat like heart, liver, legs and herring. In
vegetable sources, it occurs in spinach, cauliflower and whole
grain but in a concentration lower as compared with meat and fish [9]. According to a report, dietary intake of CoQ10
in humans is approximately 2-20mg/day. Based on the food
frequency study done in 1985 and 1995 by the National Food
Agency of Denmark, the intake of CoQ10 from diet was found to
be 3-5 mg/day in Denmark, 64 % of this daily CoQ10 originates
from meat consumption [10]. In Indians the dietary intake may
be 2-3 mg/day. The recommended daily intake has not yet been
determined. It is possible that an intake of 10-30 mg/day is
enough for healthy individuals. The South Asians have lower
plasma levels of CoQ10 as compared to Caucasians and Chinese
(Table 1).
Deficiency of Coenzyme Q10
Coenzyme Q10 is synthesized by all cells in healthy
individuals. The CoQ10 levels increase in first 20 years of life
and thereafter we lose the ability to synthesize CoQ10 due to
aging and deficiency develops. In addition to a decrease in
biosynthesis, other factors or situation that may affect the level
or functions of CoQ10 which include an increase in degradation
or change in membrane lipids which prevent the movement of
CoQ10. Another aspect causing CoQ10 deficiency is suboptimal
nutrient intake. As suboptimal nutrient intake impairing CoQ10
synthesis is almost universal, deficiency of any of the vitamins or
trace elements requiring CoQ10 synthesis can cause deficiency
of CoQ10. Decreased absorption of nutrients necessary for
synthesis of CoQ10 can be caused by aging, various diseases and use of
certain prescription medications like statins, betablockers,
anti-diabetics, etc.
Mechanism of Action of Coenzyme Q10 in various
Cardiovascular diseases- Improvement in Cardiac bioenergetics
- Free Radical Scavenger and antioxidant action
- Improvement in endothelial function and vasodilation
- Membrane stabilization action
- Preservation of myocardial Na+/K+ ATPase pump
- Anti-viscosity effect.
Coenzyme Q10: Improvement in Cardiac bioenergetics
Cardiac contraction usually happens after the release of Ca2+
from the sarcoplasmic reticulum (SR) which leads to activation of
contractile proteins actin and myosin. During diastole, cytosolic
Ca2+ re-sequesters in the SR. The cardiac contraction and reuptake
of Ca2+in the SR is an energy dependent process requiring
ATP. Myocardial relaxation that is dependent on active Ca2+
uptake by sarcoplasmic reticulum is an active process. Rather,
this step requires more energy in the form of ATP. In cases of
cardiac failure, changes in Ca2+ transport and metabolism have
been found [11]. Myocardial failure may be related to decreased
production of energy by the mitochondria. There is a decrease
in availability of energy for Ca2+ uptake in SR (diastolic failure)
and for delivery to the contractile apparatus impairing cross
bridge cycling (systolic failure). As CoQ10 participates in the
mitochondrial transport of electrons from organic substrates like
NADH and FADH2 to oxygen in the respiratory chain which leads
to the production of energy, it has a role in providing energy for
the functioning of the failing and energy depleted heart.
Coenzyme Q10: Antioxidant Action
Reactive oxygen species (ROS) negatively impact various
vascular diseases like atherosclerosis, hypertension and
diabetes mellitus and as well as in acute conditions such as
hypoxia-reoxygenation states. Clinically one of the most common
enzymatic sources of ROS is Xanthine Oxidase (XO) which is
found to be elevated in atherosclerosis. Vascular dysfunction
which is usually due pathophysiologic effects of ROS can occur
through multiple mechanisms like inactivation of endothelial
Nitric Oxide (NO) thereby generating peroxynitrite which results
in reduced ability of vessels to relax normally. Peroxynitrite can
damage lipid membrane and oxidize lipoproteins which can alter
signal transduction and cause cytotoxicity. Excess level of ROS
can increase platelet aggregation and adhesion and migration
of monocyte [12]. Coenzyme Q10 an effective antioxidant action
is a redox molecule which exists biochemically in both reduced
form (ubiquinol) as well as oxidized (ubiquinone) form in
biological tissues. In most cell membranes, enzymes have been defined that can convert ubiquinone to ubiquinol and vice-versa.
Because of its important role in mitochondrial and membrane
functions, the redox state of CoQ (ubiquinol/ubiquinone ratio)
has been suggested to be a useful biomarker of oxidative stress
[13]. In the reduced form, CoQ10 holds electrons loosely which
it can give up easily neutralizing free radicals. Reduced form of
CoQ10 displays the strongest antioxidant action. Various clinical
studies have shown that the biomarkers of oxidative stress are
decreased after CoQ10 supplementation. CoQ10 acts mainly in
the mitochondria wherein its primary function is generation of
ATP during the process of which few ROS are generated. It helps
to quench the excessive free radicals generated that threaten
cellular components such as DNA, RNA and cell membranes.
In cells, CoQ is located in the middle of the phospholipid
bilayer of various membranes; however, the relative amount
varies in different organelles [14].
Coenzyme Q10: Improved Endothelial Function & Vasodilation
Increased inactivation of NO by oxygen free radicals
contributes to endothelial dysfunction in patients with
coronary artery disease (CAD). Extracellular Superoxide
Dismutase (ecSOD) is a major antioxidant present in the
vessel wall is a principal regulator of endothelium-derived
nitric oxide oactivity [15]. In patients with CAD, the activity
of endothelium-bound ecSOD is severely reduced resulting in
decreased vasodilation [16]. A clinical study has demonstrated
that 1 month supplementation of 300 mg/day significantly
improved endothelium dependent vasodilation and increased
levels of ecSOD which is attributed to the capability of CoQ10 by
counteracting NO inactivation [17].
Coenzyme Q10 : Reduction in Pro-inflammatory Cytokines
Circulating levels of tumor necrosis factor (TNF-α)
interleukin-6 (IL-6) and C-reactive protein (CRP) have been
linked with the risk of primary and recurrent myocardial
infarction and death increases with. Various studies have
highlighted important role of inflammatory mediators in the
development of heart failure and acute myocardial infarction
(AMI) and therefore several strategies are designed to
counterbalance the different aspects of inflammatory response.
In patients with CAD, supplementation of 300 mg/day of CoQ10
resulted in decrease in inflammatory markers like IL-6 & TNF-α.
Anti-inflammatory effects of CoQ10 are due to the reduction
of nuclear factor-kB (NF- kB). Thus, CoQ10 acts by altering the
immune response [18].
Coenzyme Q10 : Role in Management of Heart FailureClinical background
Heart Failure (HF) a complex multifactorial syndrome
is usually characterized by mechanical dysfunction of the myocardium and the inability of the heart to supply adequate
amount of blood to meet the perfusion and metabolic needs
of the body. Defects in bioenergetics, abnormalities of calcium
homeostasis, altered signal transduction pathways, increased
preload and afterload and neurohormonal dysregulation are
major pathogenic factors leading to myocardial dysfunction in
HF. In order to support both electrical and mechanical activities
of the heart like contraction and diastolic relaxation, continuous
supply of energy is needed. This requirement is fulfilled by the
daily synthesis of approximately 30 kg of adenosine triphosphate
(ATP) a high-energy molecule, produced mainly by mitochondrial
oxidative phosphorylation. Energy deficits in the cardiac tissue
have been reported in HF due to alteration in all components
of cardiac energetics. Therefore, improvement in myocardial
energetics becomes a promising approach to the treatment of HF
[19]. Over the last few decades, clinical and experimental studies
have provided substantial evidences highlighting the role of
enhanced oxidative stress in HF. Excessive ROS causes cellular
dysfunction, protein and lipid peroxidation, DNA damage and
can lead to irreversible cell damage and death, which have
been implicated in a wide range of pathological cardiovascular
conditions. ROS can directly affect contractile function by
modifying proteins important for excitation-contraction
coupling. Moreover, ROS can also activate a broad variety
of hypertrophy signaling kinases and transcription factors
thereby mediating apoptosis. They also stimulate proliferation
of cardiac fibroblasts and activate the matrix metalloproteinase
(MMPs), leading to remodeling of the extracellular matrix.
These cellular events are involved in the development and
progression of maladaptive myocardial remodeling and failure
[20]. Potential sources of ROS include infiltrating inflammatory
cells, mitochondria, xanthine oxidase and NADPH oxidases.
Excessive mitochondrial-derived cardiomyocyte ROS generation
has been demonstrated in experimental models of CHF, and may
be especially important for contractile dysfunction in advanced
CHF. An elevation of xanthine oxidaseactivity and expression
has also been reported in both human end stage CHF and
canine rapid pacing-induced CHF, with the suggestion that this
contributes to contractile dysfunction [21] (Figure 3).
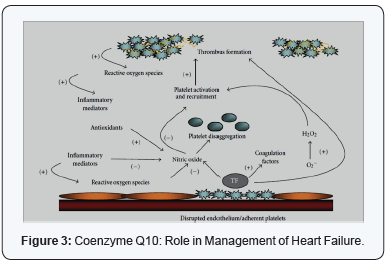
In the setting of cardiovascular disease, oxidative stress
is an important mediator of both dysfunctional endothelium
dependent vasodilation and abnormal platelet function.
Superoxide anion one of the important sources of oxidative
stresses, has direct effects, and thus limits the biological activity
of NO. Excessive production of vascular superoxide drives further
platelet activation and recruitment leading to greater thrombus
formation. The occurrence of superficial intimal injury caused
by endothelial denudation and deep intimal injury caused
by plaque rupture expose collagen and Tissue Factor (TF) to
platelets. Local platelet activation stimulates further additional
platelet recruitment and thrombus formation by supporting
cell-surface thrombin formation and releasing potent platelet
agonists such as adenosine diphosphate (ADP), serotonin,
and thromboxane A2. Platelets aggregate via the binding of
bivalent fibrinogen to GP IIb/IIIa resulting in the formation of
a thrombus. NO release from the platelet influences platelet
recruitment to the growing thrombus and impaired plateletderived
NO release which is likely to be associated with acute
coronary and stroke syndromes. Antioxidants by scavenging
of reactive oxygen species indirectly inhibit platelets. Despite
the different subcellular locations of water-soluble and lipidsoluble
antioxidants, these antioxidant pathways in platelets are
closely linked. Antioxidants indirectly inhibit platelets through
the metabolism of ROS, many of which alter platelet function.
Cardiovascular disease and acute coronary syndrome have been
linked Inflammation [22].
Coenzyme Q10 Deficiencies: Link with HF
Recent evidence suggests a role for CoQ10 is a predictor
of outcomes and also as an adjunctive clinical therapy and
therefore supplementation is routine in some countries, such as
Japan. Reduced levels of specific oxidative phosphorylation and
respiratory enzyme activities along with reduced energy reserve
in HF are considered as some of contributing factors in the
progression of disease. Low levels of CoQ10 concentration was
found in 70–75% of patients with mitral stenosis or insufficiency,
aortic stenosis or insufficiency, atrial septal defects, ventricular
septal defects and diabetic cardiomyopathy [23]. Myocardial
depletion of CoQ10 has been observed in HFpatients and the
severity of this deficiency has been found to correlate with the
severity of symptoms, in patients with NYHA class IV having
significantly lower CoQ10 in endomyocardial biopsy samples
than those in NYHA class I. In patients with cardiomyopathy this
myocardial CoQ10 deficiency can be reversed by CoQ10 therapy
[24]. In a recent observational study done in 236 subjects with
heart failure, it was found that levels of CoQ10, but not statin
therapy (known to lower CoQ10 in HF) were an independent
predictor of total mortality. This CoQ10 deficiency can be
detrimental to the long-term prognosis of CHF, and hence there is a rationale for controlled interventional studies with CoQ10
[25]. The concentration of CoQ10 is higher in the ventricles as
compared to the atrium which presumably is ascribed to greater
work burden of the ventricles and resultant greater need of
energy. The concentration of CoQ10 in the normal myocardium
has been measured as 0.42 μg/mg dry weight. In relation to this
norm the first studies of CoQ10 concentration in plasma and
myocardium showed that majority of patients with CHF had
levels of CoQ10 that were below the normal and that the lower
levels of CoQ10 occurred in conjunction with more severe stages
of CHF (NYHA III-IV) as compared with lesser severe degrees
(NYHA I-II) of CHF and with healthy persons. This correlation
between NYHA categories and CoQ10 concentration in plasma
and myocardium is thought to be independent of the underlying
cause, since this correlation is also found in other types of CHF
[26].
Summing Up: Action of Coenzyme Q10 in Heart Failure
Key substance in biological energy production (ATP), needed
for both muscle contraction and relaxation.
Trials in Heart Failure
From a meta-analysis of a main placebo controlled trial
on CoQ10 concluded that the scores for various parameters of
cardiac function was significantly better for patients given CoQ10
than for patients given placebo. An average 73% of patients
treated with CoQ10 displayed improved cardiac output, 76%
had increased stroke volume, and cardiac index was improved
in 87%, diastolic index in 88% and ejection fraction in 92% [26].
Diastolic dysfunction which is mainly due to severe
thickening of the left ventricle is one of the earliest identifiable
signs of myocardial failure accounting for 30 - 49% of heart
failure cases. In patients treated with 200 mg per day of CoQ10,
inter-ventricular septal thickness improved significantly
improving symptoms of fatigue and dyspnea with no side effects
noted [27] (Figure 4).

A meta-analysis of CoQ10 in HF there was a 3.7%
improvement in ejection fraction. Cardiac output was found to
increase by 0.28 L/min. There was a trend toward an increase in
SV. Stroke index increased an average of 5.68 mL/m2 [28].
In another multicenter trial in 1,113 CHF patients, 50-150
mg/day of CoQ10 was given for 3 months (78% of patients
received 100 mg/day). The proportion of patients with
improvement in clinical signs and symptoms were as follows:
sweating 82.4%; jugular reflex 81.5%; cyanosis 81%; pulmonary
rales 78.4%; edema 76.9%; palpitations 75.7%; vertigo 73%;
arrhythmia 62%; insomnia 60.2%; dyspnea 54.2%; nocturia
50.7%; and enlargement of liver area 49.3%. Fifty four percent
of patients had improvement of at least 3 symptoms. Moreover,
28.8% of patients entered as NYHA class III improved in to
score class II and 89.7% of patients entered in as NYHA class II
improved in score to class I [29].
Q-SYMBIO Study: Alleviating Heart Failure with Coenzyme Q10
Q-SYMBIO (SYMptoms, BIOmarker) study was initiated as
a result of encouraging effects of CoQ10 in heart failure and
need for its further research. It was a multinational, randomized
double blind, placebo controlled trial the abstract data of which
was recently published in European Society of Cardiology Heart
Failure Summit (2013). The aim of the study was to determine
whether CoQ10 supplementation would improve survival rate
and whether CoQ10 has a potential of risk reduction and to
prevent complications when used as a regular supplement. 420
patients with heart failure (NYHA class III and IV) receiving
current pharmacological therapy were randomly assigned in
parallel groups to CoQ10 (100mg) thrice daily versus placebo.
The primary long term endpoint of the study was the time to
first Major Adverse Cardiac Event (MACE) including unplanned
hospitalization due to worsening of heart failure, cardiovascular death, urgent cardiovascular transplantation and mechanical
support. CoQ10 group, after three months showed reduced level
of N-terminal pro-brain natriuretic peptide (NT-pro BNP), while
significant improvement of the NYHA Class (p = 0.047) was
observed at the end of two years. The primary endpoint was
reached by 29 patients in the CoQ10 group, as compared with 55
patients in the placebo group. All-cause mortality was also found
to be lower in the CoQ10 group (18 patients) versus placebo (36
patients). There were fewer adverse events in the CoQ10 group
compared to the placebo group (p = 0.073). Thus the patients
treated with CoQ10had reduced hospital admission rates for
worsening HF and lower cardiovascular death both of which may
reflect a significant improvement in cardiac function. Results of
the Q-SYMBIO study showed that CoQ10 fulfills various criteria
of an obvious adjunct in patients with symptomatic HF along
with standard therapy [30].
Clinical Implication of Coenzyme Q10 usages in patients with end-stage heart failure waiting for heart transplantation
A clinical study done in 27 patients with end-stage heart
failure waiting for heart transplantation with evident symptoms
of fatigue, nocturia and dyspnea showed a great improvement
after supplementation with 60 mg of CoQ10 daily for 3 months.
The patient showed significant improvement in clinical
symptoms, functional status and quality of life of patients. This
improvement was because external correction of CoQ10 levels
can presumably restore the mitochondrial bioenergetics and
exert an antioxidant effect, which increases the oxygen delivery
to the striated skeletal muscle. The findings of this study support
the efficacy of CoQ10 treatment on symptoms in patients
suffering from end-stage heart failure. Hence, CoQ10 should be
considered as an optional addition to regular medical regimen
for the management of end stage heart failure [31].
Coenzyme Q10 and Statins
Patients with cardiovascular diseases are usually
prescribed statins for primary as well as secondary
prevention of future cardiac events.
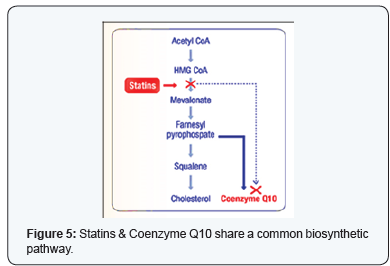
CStatin supplementation leading to deficiency of CoQ10
Statins being an effective class of drug for reducing low
density lipoprotein (LDL) are also associated with beneficial
impact on cardiovascular morbidity and mortality. Statins
block the endogenous biosynthesis of cholesterol as well as
Coenzyme Q10 by inhibiting the enzyme HMG CoA reductase,
resulting in Coenzyme Q10 deficiency. The resulting reduction
in blood CoQ10 level is due to the partially shared biosynthetic
mevalonate pathway of CoQ10 and cholesterol. Statins are
known to reduce cholesterol/LDL levels by inhibiting HMG-CoA
reductase, they can as well lower serum levels of coenzyme Q10
up to 40%. This results into depletion of CoQ10levels in patients
with heart failure using statins; this may lead to significant
harmful effects which can be negated by oral CoQ10 (Figure 5).
Benefits of Stains & Coenzyme Q10 in patients with Chronic Heart Failure (CHF):
Statins reduce the already low levels of CoQ10 present in
patients with Heart failure. Coenzyme Q10 and Statins have the
potential to improve cardiac function in two possible ways:
- Coenzyme Q10 supplementation along with statins replace the statin depleted CoQ10 levels. CoQ10 also exerts its bioenergetic action by improving the efficiency of energy production (ATP) in the heart thereby improving the myocardial contractility. Various clinical trials have shown that CoQ10 improves functional capacity, symptoms, and QoL in patients with no significant side effects in patients with HF. Coenzyme Q10 supplementation also showed significant improvement in hemodynamic parameters like cardiac index, ejection fraction, stroke volume and end diastolic volume.
- Also the combination of CoQ10 and statin has shown synergistic action on oxidative stress through activation of the enzyme superoxide dismutase that regulates nitric oxide metabolism and thus the determining step in free radical scavenging. Low markers of oxidative stress have been linked with improved cardiac function by both statins and coenzyme Q10 [32,33].
Coenzyme Q10 is usually given as an adjuvant therapy in the
management of various CVDs. As per various clinical trials and
documented evidences, in Heart Failure the dosage of coenzyme
Q10 is 100-200 mg/day which can be increased up to 300 mg/
day. Coenzyme Q10 are usually given along with Statin in the
dose of 100 mg/day.
The relationship of beneficial effects of CoQ10 in patients
with congestive heart failure has been studied for decades. Early
studies in patients with heart failure have reported declining
myocardial level of CoQ10 with increasing severity of heart
failure. Such a decline in CoQ10 levels might be exacerbated
by concurrent treatment with statins and β-blockers, which
can further suppress endogenous synthesis of CoQ10. Pilot
clinical trials involving supplementation of CoQ10 in HF patients
reported improvement in various functional parameters
such as ejection fraction, stroke volume and cardiac output,
with minimal side effects. Most definitively, recent Q-SYMBIO
trial, a multicenter randomized placebo-controlled trial, has
demonstrated the beneficial impact of supplemental CoQ10 on
hard end points in HF. Thus, in conclusion, increasing evidences
suggests that adjuvant supplementation of CoQ10 may be a
useful option for effective management of heart failure, with the
advantage of excellent clinical tolerance—reflecting its status as
an essential physiological cofactor.



Comments
Post a Comment